Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease
- PMID: 22811541
- PMCID: PMC3457301
- DOI: 10.1128/JVI.01439-12
Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease
Abstract
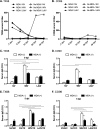
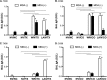
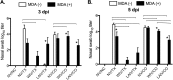
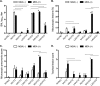
Control of swine influenza A virus (IAV) in the United States is hindered because inactivated vaccines do not provide robust cross-protection against the multiple antigenic variants cocirculating in the field. Vaccine efficacy can be limited further for vaccines administered to young pigs that possess maternally derived immunity. We previously demonstrated that a recombinant A/sw/Texas/4199-2/1998 (TX98) (H3N2) virus expressing a truncated NS1 protein is attenuated in swine and has potential for use as an intranasal live attenuated influenza virus (LAIV) vaccine. In the present study, we compared 1 dose of intranasal LAIV with 2 intramuscular doses of TX98 whole inactivated virus (WIV) with adjuvant in weanling pigs with and without TX98-specific maternally derived antibodies (MDA). Pigs were subsequently challenged with wild-type homologous TX98 H3N2 virus or with an antigenic variant, A/sw/Colorado/23619/1999 (CO99) (H3N2). In the absence of MDA, both vaccines protected against homologous TX98 and heterologous CO99 shedding, although the LAIV elicited lower hemagglutination inhibition (HI) antibody titers in serum. The efficacy of both vaccines was reduced by the presence of MDA; however, WIV vaccination of MDA-positive pigs led to dramatically enhanced pneumonia following heterologous challenge, a phenomenon known as vaccine-associated enhanced respiratory disease (VAERD). A single dose of LAIV administered to MDA-positive pigs still provided partial protection from CO99 and may be a safer vaccine for young pigs under field conditions, where dams are routinely vaccinated and diverse IAV strains are in circulation. These results have implications not only for pigs but also for other influenza virus host species.
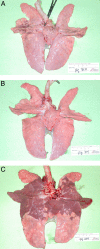
Figures
References
-
- Choi YK, Goyal SM, Joo HS. 2004. Evaluation of transmission of swine influenza type A subtype H1N2 virus in seropositive pigs. Am. J. Vet. Res. 65:303–306 - PubMed
-
- Gauger PC, et al. 2011. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29:2712–2719 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

