Transforming growth factor Beta 1 stimulates profibrotic activities of luteal fibroblasts in cows
- PMID: 22811573
- PMCID: PMC5597442
- DOI: 10.1095/biolreprod.112.100735
Transforming growth factor Beta 1 stimulates profibrotic activities of luteal fibroblasts in cows
Abstract
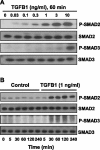
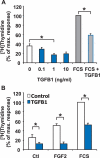
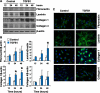
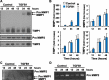
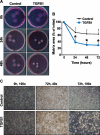
Luteolysis is characterized by angioregression, luteal cell apoptosis, and remodeling of the extracellular matrix characterized by deposition of collagen 1. Transforming growth factor beta 1 (TGFB1) is a potent mediator of wound healing and fibrotic processes through stimulation of the synthesis of extracellular matrix components. We hypothesized that TGFB1 stimulates profibrotic activities of luteal fibroblasts. We examined the actions of TGFB1 on luteal fibroblast proliferation, extracellular matrix production, floating gel contraction, and chemotaxis. Fibroblasts were isolated from the bovine corpus luteum. Western blot analysis showed that luteal fibroblasts expressed collagen 1 and prolyl 4-hydroxylase but did not express markers of endothelial or steroidogenic cells. Treatment of fibroblasts with TGFB1 stimulated the phosphorylation of SMAD2 and SMAD3. [(3)H]thymidine incorporation studies showed that TGFB1 caused concentration-dependent reductions in DNA synthesis in luteal fibroblasts and significantly (P < 0.05) reduced the proliferative effect of FGF2 and fetal calf serum. However, TGFB1 did not reduce the viability of luteal fibroblasts. Treatment of luteal fibroblasts with TGFB1 induced the expression of laminin, collagen 1, and matrix metalloproteinase 1 as determined by Western blot analysis and gelatin zymography of conditioned medium. TGFB1 increased the chemotaxis of luteal fibroblasts toward fibronectin in a transwell system. Furthermore, TGFB1 increased the fibroblast-mediated contraction of floating bovine collagen 1 gels. These results suggest that TGFB1 contributes to the structural regression of the corpus luteum by stimulating luteal fibroblasts to remodel and contract the extracellular matrix.
Figures




References
-
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW.. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 2000; 80:1–29. - PubMed
-
- Duncan WC, McNeilly AS, Illingworth PJ.. The effect of luteal “rescue” on the expression and localization of matrix metalloproteinases and their tissue inhibitors in the human corpus luteum. J Clin Endocrinol Metab 1998; 83:2470–2478. - PubMed
-
- Smith MF, Ricke WA, Bakke LJ, Dowb MPD, Smith GW.. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol 2002; 191:45–56. - PubMed
-
- Stocco C, Telleria C, Gibori G.. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 2007; 28:117–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

