Ras-driven transcriptome analysis identifies aurora kinase A as a potential malignant peripheral nerve sheath tumor therapeutic target
- PMID: 22811580
- PMCID: PMC3902639
- DOI: 10.1158/1078-0432.CCR-12-1072
Ras-driven transcriptome analysis identifies aurora kinase A as a potential malignant peripheral nerve sheath tumor therapeutic target
Abstract
Purpose: Patients with neurofibromatosis type 1 (NF1) develop malignant peripheral nerve sheath tumors (MPNST), which are often inoperable and do not respond well to current chemotherapies or radiation. The goal of this study was to use comprehensive gene expression analysis to identify novel therapeutic targets.
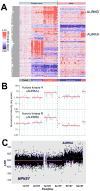
Experimental design: Nerve Schwann cells and/or their precursors are the tumorigenic cell types in MPNST because of the loss of the NF1 gene, which encodes the RasGAP protein neurofibromin. Therefore, we created a transgenic mouse model, CNP-HRas12V, expressing constitutively active HRas in Schwann cells and defined a Ras-induced gene expression signature to drive a Bayesian factor regression model analysis of differentially expressed genes in mouse and human neurofibromas and MPNSTs. We tested functional significance of Aurora kinase overexpression in MPNST in vitro and in vivo using Aurora kinase short hairpin RNAs (shRNA) and compounds that inhibit Aurora kinase.
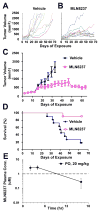
Results: We identified 2,000 genes with probability of linkage to nerve Ras signaling of which 339 were significantly differentially expressed in mouse and human NF1-related tumor samples relative to normal nerves, including Aurora kinase A (AURKA). AURKA was dramatically overexpressed and genomically amplified in MPNSTs but not neurofibromas. Aurora kinase shRNAs and Aurora kinase inhibitors blocked MPNST cell growth in vitro. Furthermore, an AURKA selective inhibitor, MLN8237, stabilized tumor volume and significantly increased survival of mice with MPNST xenografts.
Conclusion: Integrative cross-species transcriptome analyses combined with preclinical testing has provided an effective method for identifying candidates for molecular-targeted therapeutics. Blocking Aurora kinases may be a viable treatment platform for MPNST.
©2012 AACR.
Conflict of interest statement
Figures



References
-
- Katz D, Lazar A, Lev D. Malignant peripheral nerve sheath tumour (MPNST): the clinical implications of cellular signalling pathways. Expert Rev Mol Med. 2009;11:e30. - PubMed
-
- Bottillo I, Ahlquist T, Brekke H, Danielsen SA, van den Berg E, Mertens F, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693–701. - PubMed
-
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:663–664. - PubMed
-
- DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (Type 1) neurofibromatosis. Cell. 1992;69:265–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

