Restriction of Retroviral Replication by Tetherin/BST-2
- PMID: 22811908
- PMCID: PMC3395152
- DOI: 10.1155/2012/424768
Restriction of Retroviral Replication by Tetherin/BST-2
Abstract
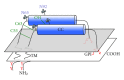
Tetherin/BST-2 is an important host restriction factor that limits the replication of HIV and other enveloped viruses. Tetherin is a type II membrane glycoprotein with a very unusual domain structure that allows it to engage budding virions and retain them on the plasma membrane of infected cells. Following the initial report identifying tetherin as the host cell factor targeted by the HIV-1 Vpu gene, knowledge of the molecular, structural, and cellular biology of tetherin has rapidly advanced. This paper summarizes the discovery and impact of tetherin biology on the HIV field, with a focus on recent advances in understanding its structure and function. The relevance of tetherin to replication and spread of other retroviruses is also reviewed. Tetherin is a unique host restriction factor that is likely to continue to provide new insights into host-virus interactions and illustrates well the varied ways by which host organisms defend against viral pathogens.
Figures

References
-
- Strebel K, Klimkait T, Martin MA. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241(4870):1221–1223. - PubMed
-
- Terwilliger EF, Godin B, Sodroski JG, Haseltine WA. Construction and use of a replication-competent human immunodeficiency virus (HIV-1) that expresses the chloramphenicol acetyltransferase enzyme. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(10):3857–3861. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
