Regulation of signal transduction and role of platelets in liver regeneration
- PMID: 22811921
- PMCID: PMC3395153
- DOI: 10.1155/2012/542479
Regulation of signal transduction and role of platelets in liver regeneration
Abstract
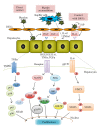
Among all organs, the liver has a unique regeneration capability after sustaining injury or the loss of tissue that occurs mainly due to mitosis in the hepatocytes that are quiescent under normal conditions. Liver regeneration is induced through a cascade of various cytokines and growth factors, such as, tumor necrosis factor alpha, interleukin-6, hepatocyte growth factor, and insulin-like growth factor, which activate nuclear factor κB, signal transducer and activator of transcription 3, and phosphatidyl inositol 3-kinase signaling pathways. We previously reported that platelets can play important roles in liver regeneration through a direct effect on hepatocytes and collaborative effects with the nonparenchymal cells of the liver, including Kupffer cells and liver sinusoidal endothelial cells, which participate in liver regeneration through the production of various growth factors and cytokines. In this paper, the roles of platelets and nonparenchymal cells in liver regeneration, including the associated cytokines, growth factors, and signaling pathways, are described.
Figures
References
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–65. - PubMed
-
- Fausto N. Liver regeneration: from laboratory to clinic. Liver Transplantation. 2001;7(10):835–844. - PubMed
-
- Pahlavan PS, Feldmann RE, Jr., Zavos C, Kountouras J. Prometheus’ challenge: molecular, cellular and systemic aspects of liver regeneration. Journal of Surgical Research. 2006;134(2):238–251. - PubMed
-
- Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. Journal of the American College of Surgeons. 1999;188(3):304–309. - PubMed
-
- Topal B, Kaufman L, Aerts R, Penninckx F. Patterns of failure following curative resection of colorectal liver metastases. European Journal of Surgical Oncology. 2003;29(3):248–253. - PubMed
LinkOut - more resources
Full Text Sources

