Targeting iron assimilation to develop new antibacterials
- PMID: 22812521
- PMCID: PMC3434712
- DOI: 10.1517/17460441.2012.708335
Targeting iron assimilation to develop new antibacterials
Abstract
Introduction: Since the first application of antibiotics to treat bacterial infections, the development and spread of resistance has been a persistent threat. An ever evolving pipeline of next-generation therapeutics is required for modern medicine to remain one step ahead of pathogens.
Areas covered: This review describes recent efforts to develop drugs that interrupt the assimilation of iron by bacteria: a process that is vital to cellular homeostasis and is not currently targeted by antibiotics used in the clinic. This review also covers the mechanisms by which bacteria acquire iron for their environment, and details efforts to intervene in these processes, using small molecule inhibitors that target key steps in these pathways, with a special emphasis on recent advances published during the 2010 - 2012 period.
Expert opinion: For decades, the routes used by bacteria to assimilate iron from host and environmental settings have been the subject of intense study. While numerous investigations have identified inhibitors of these pathways, many have stopped short of translating the in vitro results to in vivo proof of concept experiments. The extension of preliminary findings in this manner will significantly increase the impact of the field.
Figures

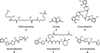

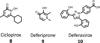
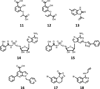

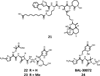

References
-
- Power E. Impact of antibiotic restrictions: the pharmaceutical perspective. Clinical Microbiology and Infection. 2006 Aug;12:25–34. - PubMed
-
- Norrby R, Powell M. The bacterial challenge: time to react. Stockholm: European Centre for Disease Prevention and Control; 2009.
-
- Choffnes ER, Relman DA, Mack A. Antibiotic Resistance: Implications for global health and novel intervention strategies. Washington, D.C.: National Academies Press; 2010. - PubMed
-
- America IDSo. Bad bugs, no drugs: as antibiotic discovery stagnates… a public health crisis brews. Alexandria, VA: Infectious Disease Society of America; 2004.
-
-
Gilbert DN, Guidos RJ, Boucher HW, et al. The 10 × '20 Initiative: Pursuing a Global Commitment to Develop 10 New Antibacterial Drugs by 2020. Clinical Infectious Diseases. 2010 Apr 15;50(8):1081–1083. Description of the Infectious Disease Society of America's intiative to bring 10 new antibacterial compounds to the clinic by 2020.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
