Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study
- PMID: 22812594
- PMCID: PMC3524372
- DOI: 10.1111/j.1464-5491.2012.03750.x
Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study
Abstract
Aims: Whether long-term cardiovascular risk is reduced by the Diabetes Prevention Program interventions is unknown. The aim of this study was to determine the long-term differences in cardiovascular disease risk factors and the use of lipid and blood pressure medications by the original Diabetes Prevention Program intervention group.
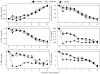
Methods: This long-term follow-up (median 10 years, interquartile range 9.0-10.5) of the three-arm Diabetes Prevention Program randomized controlled clinical trial (metformin, intensive lifestyle and placebo), performed on 2766 (88%) of the Diabetes Prevention Program participants (who originally had impaired glucose tolerance), comprised a mean of 3.2 years of randomized treatment, approximately 1-year transition (during which all participants were offered intensive lifestyle intervention) and 5 years follow-up (Diabetes Prevention Program Outcomes Study). During the study, participants were followed in their original groups with their clinical care being provided by practitioners outside the research setting. The study determined lipoprotein profiles and blood pressure and medication use annually.
Results: After 10 years' follow-up from Diabetes Prevention Program baseline, major reductions were seen for systolic (-2 to -3) and diastolic (-6 to -6.5 mmHg) blood pressure, and for LDL cholesterol (-0.51 to -0.6 mmol/l) and triglycerides (-0.23 to -0.25 mmol/l) in all groups, with no between-group differences. HDL cholesterol also rose significantly (0.14 to 0.15 mmol/l) in all groups. Lipid (P = 0.01) and blood pressure (P = 0.09) medication use, however, were lower for the lifestyle group during the Diabetes Prevention Program Outcomes Study.
Conclusion: Overall, intensive lifestyle intervention achieved, with less medication, a comparable long-term effect on cardiovascular disease risk factors, to that seen in the metformin and placebo groups.
Trial registration: ClinicalTrials.gov NCT00038727.
© 2012 The Authors. Diabetic Medicine © 2012 Diabetes UK.
Conflict of interest statement
TJO has been a consultant for Astra Zeneca. RG has speaker honoraria from Merck, GSK and Daiichi-Sankoyo; research grants from Abbott, GSK and Roche; and is on the Consultant Board of GSK, Daiichi-Sankoyo and Pfizer. MT, EB-C, SF, KM, SM, MM, RR, CS, HS and KW have nothing to declare.
Figures


References
-
- Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary heart disease risk and impaired glucose tolerance: the Whitehall Study. Lancet. 1980;8183:1373–1376. - PubMed
-
- Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. - PubMed
-
- Tuomilehto J, Lindström J, Eriksson JG, et al. for the Finnish Diabetes Prevention Study Group. N Engl J Med. 2001;344:1343–1350. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

