Pupillometric analysis for assessment of gene therapy in Leber Congenital Amaurosis patients
- PMID: 22812667
- PMCID: PMC3526436
- DOI: 10.1186/1475-925X-11-40
Pupillometric analysis for assessment of gene therapy in Leber Congenital Amaurosis patients
Abstract
Background: Objective techniques to assess the amelioration of vision in patients with impaired visual function are needed to standardize efficacy assessment in gene therapy trials for ocular diseases. Pupillometry has been investigated in several diseases in order to provide objective information about the visual reflex pathway and has been adopted to quantify visual impairment in patients with Leber Congenital Amaurosis (LCA). In this paper, we describe detailed methods of pupillometric analysis and a case study on three Italian patients affected by Leber Congenital Amaurosis (LCA) involved in a gene therapy clinical trial at two follow-up time-points: 1 year and 3 years after therapy administration.
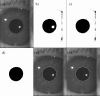
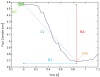
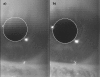
Methods: Pupillary light reflexes (PLR) were measured in patients who had received a unilateral subretinal injection in a clinical gene therapy trial. Pupil images were recorded simultaneously in both eyes with a commercial pupillometer and related software. A program was generated with MATLAB software in order to enable enhanced pupil detection with revision of the acquired images (correcting aberrations due to the inability of these severely visually impaired patients to fixate), and computation of the pupillometric parameters for each stimulus. Pupil detection was performed through Hough Transform and a non-parametric paired statistical test was adopted for comparison.
Results: The developed program provided correct pupil detection also for frames in which the pupil is not totally visible. Moreover, it provided an automatic computation of the pupillometric parameters for each stimulus and enabled semi-automatic revision of computerized detection, eliminating the need for the user to manually check frame by frame. With reference to the case study, the amplitude of pupillary constriction and the constriction velocity were increased in the right (treated eye) compared to the left (untreated) eye at both follow-up time-points, showing stability of the improved PLR in the treated eye.
Conclusions: Our method streamlined the pupillometric analyses and allowed rapid statistical analysis of a range of parameters associated with PLR. The results confirm that pupillometry is a useful objective measure for the assessment of therapeutic effect of gene therapy in patients with LCA.
Trial registration: ClinicalTrials.gov NCT00516477.
Figures
References
-
- Banin E, Bandah-Rozenfeld D, Obolensky A, Cideciyan AV, Aleman TS, Marks-Ohana D, Sela M, Boye S, Sumaroka A, Roman AJ. et al.Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther. 2010;21:1749–1757. doi: 10.1089/hum.2010.047. - DOI - PubMed
-
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

