Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium
- PMID: 22814379
- PMCID: PMC3412036
- DOI: 10.1073/pnas.1204302109
Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium
Abstract
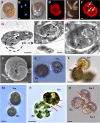
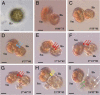
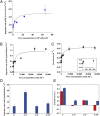
Survival of free-living and symbiotic dinoflagellates (Symbiodinium spp.) in coral reefs is critical to the maintenance of a healthy coral community. Most coral reefs exist in oligotrophic waters, and their survival strategy in such nutrient-depleted waters remains largely unknown. In this study, we found that two strains of Symbiodinium spp. cultured from the environment and acquired from the tissues of the coral Alveopora japonica had the ability to feed heterotrophically. Symbiodinium spp. fed on heterotrophic bacteria, cyanobacteria (Synechococcus spp.), and small microalgae in both nutrient-replete and nutrient-depleted conditions. Cultured free-living Symbiodinium spp. displayed no autotrophic growth under nitrogen-depleted conditions, but grew when provided with prey. Our results indicate that Symbiodinium spp.'s mixotrophic activity greatly increases their chance of survival and their population growth under nitrogen-depleted conditions, which tend to prevail in coral habitats. In particular, free-living Symbiodinium cells acquired considerable nitrogen from algal prey, comparable to or greater than the direct uptake of ammonium, nitrate, nitrite, or urea. In addition, free-living Symbiodinium spp. can be a sink for planktonic cyanobacteria (Synechococcus spp.) and remove substantial portions of Synechococcus populations from coral reef waters. Our discovery of Symbiodinium's feeding alters our conventional views of the survival strategies of photosynthetic Symbiodinium and corals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. - PubMed
-
- Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. - PubMed
-
- Pennisi E. Reefs in trouble: Spawning for a better life. Science. 2007;318:1712–1717. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

