Integrating the landscape epidemiology and genetics of RNA viruses: rabies in domestic dogs as a model
- PMID: 22814380
- PMCID: PMC3526958
- DOI: 10.1017/S003118201200090X
Integrating the landscape epidemiology and genetics of RNA viruses: rabies in domestic dogs as a model
Abstract
Landscape epidemiology and landscape genetics combine advances in molecular techniques, spatial analyses and epidemiological models to generate a more real-world understanding of infectious disease dynamics and provide powerful new tools for the study of RNA viruses. Using dog rabies as a model we have identified how key questions regarding viral spread and persistence can be addressed using a combination of these techniques. In contrast to wildlife rabies, investigations into the landscape epidemiology of domestic dog rabies requires more detailed assessment of the role of humans in disease spread, including the incorporation of anthropogenic landscape features, human movements and socio-cultural factors into spatial models. In particular, identifying and quantifying the influence of anthropogenic features on pathogen spread and measuring the permeability of dispersal barriers are important considerations for planning control strategies, and may differ according to cultural, social and geographical variation across countries or continents. Challenges for dog rabies research include the development of metapopulation models and transmission networks using genetic information to uncover potential source/sink dynamics and identify the main routes of viral dissemination. Information generated from a landscape genetics approach will facilitate spatially strategic control programmes that accommodate for heterogeneities in the landscape and therefore utilise resources in the most cost-effective way. This can include the efficient placement of vaccine barriers, surveillance points and adaptive management for large-scale control programmes.
Figures
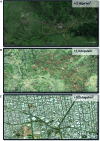
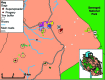
Similar articles
-
Response to a rabies epidemic, Bali, Indonesia, 2008-2011.Emerg Infect Dis. 2013 Apr;19(4):648-51. doi: 10.3201/eid1904.120380. Emerg Infect Dis. 2013. PMID: 23632033 Free PMC article.
-
Mathematical modelling and phylodynamics for the study of dog rabies dynamics and control: A scoping review.PLoS Negl Trop Dis. 2021 May 27;15(5):e0009449. doi: 10.1371/journal.pntd.0009449. eCollection 2021 May. PLoS Negl Trop Dis. 2021. PMID: 34043640 Free PMC article.
-
Phylogenetic analysis of rabies viruses from Burkina Faso, 2007.Zoonoses Public Health. 2010 Dec;57(7-8):e42-6. doi: 10.1111/j.1863-2378.2009.01291.x. Zoonoses Public Health. 2010. PMID: 19968849
-
Genome-based local dynamics of canine rabies virus epidemiology, transmission, and evolution in Davao City, Philippines, 2018-2019.Infect Genet Evol. 2021 Aug;92:104868. doi: 10.1016/j.meegid.2021.104868. Epub 2021 Apr 17. Infect Genet Evol. 2021. PMID: 33878454
-
Successful strategies implemented towards the elimination of canine rabies in the Western Hemisphere.Antiviral Res. 2017 Jul;143:1-12. doi: 10.1016/j.antiviral.2017.03.023. Epub 2017 Apr 4. Antiviral Res. 2017. PMID: 28385500 Free PMC article. Review.
Cited by
-
Habitat connectivity and host relatedness influence virus spread across an urbanising landscape in a fragmentation-sensitive carnivore.Virus Evol. 2022 Dec 21;9(1):veac122. doi: 10.1093/ve/veac122. eCollection 2023. Virus Evol. 2022. PMID: 36694819 Free PMC article.
-
Surveillance of Canine Rabies in the Central African Republic: Impact on Human Health and Molecular Epidemiology.PLoS Negl Trop Dis. 2016 Feb 9;10(2):e0004433. doi: 10.1371/journal.pntd.0004433. eCollection 2016 Feb. PLoS Negl Trop Dis. 2016. PMID: 26859829 Free PMC article.
-
Elucidating the phylodynamics of endemic rabies virus in eastern Africa using whole-genome sequencing.Virus Evol. 2015 Sep 10;1(1):vev011. doi: 10.1093/ve/vev011. eCollection 2015. Virus Evol. 2015. PMID: 27774283 Free PMC article.
-
Movement patterns of free-roaming dogs on heterogeneous urban landscapes: Implications for rabies control.Prev Vet Med. 2020 May;178:104978. doi: 10.1016/j.prevetmed.2020.104978. Epub 2020 Mar 31. Prev Vet Med. 2020. PMID: 32302776 Free PMC article.
-
Spatial and temporal epidemiological analysis in the Big Data era.Prev Vet Med. 2015 Nov 1;122(1-2):213-20. doi: 10.1016/j.prevetmed.2015.05.012. Epub 2015 Jun 6. Prev Vet Med. 2015. PMID: 26092722 Free PMC article. Review.
References
-
- Anderson R. M. and May R. M. (1991). Infectious Diseases of Humans: Dynamics and Control. Oxford University Press, Oxford: 126, 186–195
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

