Intralipid, a clinically safe compound, protects the heart against ischemia-reperfusion injury more efficiently than cyclosporine-A
- PMID: 22814384
- PMCID: PMC3769111
- DOI: 10.1097/ALN.0b013e3182655e73
Intralipid, a clinically safe compound, protects the heart against ischemia-reperfusion injury more efficiently than cyclosporine-A
Abstract
Background: We have recently shown that postischemic administration of intralipid protects the heart against ischemia-reperfusion injury. Here we compared the cardioprotective effects of intralipid with cyclosporine-A, a potent inhibitor of the mitochondrial permeability transition pore opening.
Methods: In vivo rat hearts or isolated Langendorff-perfused mouse hearts were subjected to ischemia followed by reperfusion with intralipid (0.5%, 1% and 2% ex-vivo, and 20% in vivo), cyclosporine-A (0.2 μM, 0.8 μM, and 1.5 μM ex- vivo and 10 mg/kg in vivo), or vehicle. The hemodynamic function, infarct size, calcium retention capacity, mitochondrial superoxide production, and phosphorylation levels of protein kinase B (Akt)/glycogen synthase kinase-3β (GSK-3β) were measured. The values are mean ± SEM.
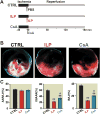
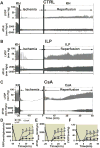
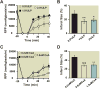
Results: Administration of intralipid at reperfusion significantly reduced myocardial infarct size compared with cyclosporine-A in vivo (infarct size/area at risk)%: 22.9 ± 2.5% vs. 35.2 ± 3.5%; P = 0.030, n = 7/group). Postischemic administration of intralipid at its optimal dose (1%) was more effective than cyclosporine-A (0.8 μM) in protecting the ex vivo heart against ischemia-reperfusion injury, as the rate pressure product at the end of reperfusion was significantly higher (mmHg · beats/min: 12,740 ± 675 [n = 7] vs. 9,203 ± 10,781 [n = 5], P = 0.024), and the infarct size was markedly smaller (17.3 ± 2.9 [n = 7] vs. 29.2 ± 2.7 [n = 5], P = 0.014). Intralipid was as efficient as cyclosporine-A in inhibiting the mitochondrial permeability transition pore opening (calcium retention capacity = 280 ± 8.2 vs. 260.3 ± 2.9 nmol/mg mitochondria protein in cyclosporine-A, P = 0.454, n = 6) and in reducing cardiac mitochondrial superoxide production. Unlike intralipid, which increased phosphorylation of Akt (6-fold) and GSK-3β (5-fold), cyclosporine-A had no effect on the activation of these prosurvival kinases.
Conclusions: Although intralipid inhibits the opening of the mitochondrial permeability transition pore as efficiently as cyclosporine-A, intralipid is more effective in reducing the infarct size and improving the cardiac functional recovery.
Figures


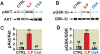

References
-
- Gorczynski RJ. Basic pharmacology of esmolol. Am J Cardiol. 1985;56:3F–13F. - PubMed
-
- Sugimoto S, Puddu PE, Monti F, Schiariti M, Campa PP, Marino B. Pretreatment with the adenosine triphosphate-sensitive potassium channel opener nicorandil and improved myocardial protection during high-potassium cardioplegic hypoxia. J Thorac Cardiovasc Surg. 1994;108:455–66. - PubMed
-
- Dorman BH, Hebbar L, Zellner JL, New RB, Houck WV, Acsell J, Nettles C, Hendrick JW, Sampson AP, Mukherjee R, Spinale FG. ATP-sensitive potassium channel activation before cardioplegia. Effects on ventricular and myocyte function. Circulation. 1998;98(II):176–83. - PubMed
-
- Cour M, Gomez L, Mewton N, Ovize M, Argaud L. Postconditioning: From the bench to bedside. J Cardiovasc Pharmacol Ther. 2011;16:117–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

