A randomised study evaluating the use of pyridoxine to avoid capecitabine dose modifications
- PMID: 22814578
- PMCID: PMC3419962
- DOI: 10.1038/bjc.2012.318
A randomised study evaluating the use of pyridoxine to avoid capecitabine dose modifications
Abstract
Background: Pyridoxine is frequently used to treat capecitabine-induced hand-foot syndrome (HFS), although the evidence of benefit is lacking. We performed a randomised placebo-controlled trial to determine whether pyridoxine could avoid the need for capecitabine dose modifications and improve outcomes.
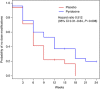
Methods: A total of 106 patients planned for palliative single-agent capecitabine (53 in each arm, 65%/35% colorectal/breast cancer) were randomised to receive either concomitant pyridoxine (50 mg po) or matching placebo three times daily.
Results: Compared with placebo, pyridoxine use was associated with an increased rate of avoiding capecitabine dose modifications (37% vs 23%, relative risk 0.59, 95% CI 0.29, 1.20, P=0.15) and fewer grade 3/4 HFS-related adverse events (9% vs 17%, odds ratio 0.51, 95% CI 0.15-1.6, P=0.26). Use of pyridoxine did not improve response rate or progression-free survival.
Conclusion: Pyridoxine may reduce the need for capecitabine dose modifications and the incidence of severe HFS, but does not impact on antitumour effect.
Figures
References
-
- Beveridge RA, Kales AN, Binder RA, Miller JA, Virts SG (1990) Pyridoxine (B6) and amelioration of hand/foot syndrome. Proc Am Soc Clin Oncol 9: 102a (abstr 393)
-
- Chalermchai T, Tantiphlachiva K, Suwanrusme H, Voravud N, Sriuranpong V (2010) Randomized trial of two different doses of pyridoxine in the prevention of capecitabine-associated palmar-plantar erythrodysesthesia. Asia-Pacific J Clin Oncol 6: 155–160 - PubMed
-
- Fabian CJ, Molina R, Slavik M, Dahlberg S, Giri S, Stephens R (1990) Pyridoxine therapy for palmar-plantar erythrodysesthesia associated with continuous 5-fluorouracil infusion. Invest New Drugs 8: 57–63 - PubMed
-
- Gressett SM, Stanford BL, Hardwicke F (2006) Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract 12: 131–141 - PubMed
-
- Kang YK, Lee SS, Yoon DH, Lee SY, Chun YJ, Kim MS, Ryu MH, Chang HM, Lee JL, Kim TW (2010) Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: results of a randomized, double-blind, placebo-controlled study. J Clin Oncol 28: 3824–3829 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical