Personalising docetaxel and G-CSF schedules in cancer patients by a clinically validated computational model
- PMID: 22814580
- PMCID: PMC3425973
- DOI: 10.1038/bjc.2012.316
Personalising docetaxel and G-CSF schedules in cancer patients by a clinically validated computational model
Abstract
Background: This study was aimed to develop a new method for personalising chemotherapeutic and granulocyte colony-stimulating factor (G-CSF) combined schedules, and use it for suggesting efficacious chemotherapy with reduced neutropenia.
Methods: Clinical data from 38 docetaxel (Doc)-treated metastatic breast cancer patients were employed for validating a new pharmacokinetic/pharmacodynamics model for Doc, combined with a mathematical model for granulopoiesis. An optimisation procedure was constructed and used for selecting improved treatment schedules.
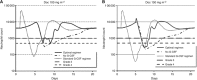
Results: The combined model accurately predicted observed nadir timing (r=0.99), grade 3 or 4 neutropenia (86% success) and neutrophil counts over time in individual patients (r=0.63), and showed robustness to CYP3A-induced variability in Doc clearance. For average patients, the predicted optimal support for the standard chemotherapy regimen, Doc 100 μg m(-2) tri-weekly, is G-CSF, 300 μg, Q1D × 3, starting day 7 post-Doc. This regimen largely moderates chemotherapy-induced neutrophil nadir and neutropenia duration. The more intensive Doc dose, 150 mg m(-2), is optimally supported by the slightly less cost-effective G-CSF 300 μg, Q1D × 4, 5 days post-Doc. The latter regimen is optimal for borderline patients (2000 neutrophils per μl) under Doc, 100-150 mg m(-2) tri-weekly.
Conclusions: The new computational method can serve for tailoring efficacious cytotoxic and supportive treatments, minimising side effects to individual patients. Prospective clinical validation is warranted.
Figures



References
-
- Agur Z, Hassin R, Levy S (2006) Optimizing chemotherapy scheduling using local search heuristics. Operations Res 54: 829–846
-
- Baker SD, Zhao M, Lee CK, Verweij J, Zabelina Y, Brahmer JR, Wolff AC, Sparreboom A, Carducci MA (2004) Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res 10(6): 1976–1983 - PubMed
-
- Bonate P (2006) Pharmacokinetic-Pharmacodynamic Modeling and Simulation. Springer Science and Business Media Inc: New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

