Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT
- PMID: 22814600
- PMCID: PMC3587727
- DOI: 10.1016/j.devcel.2012.05.014
Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT
Abstract
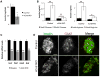
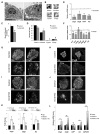
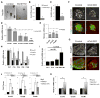
Little is known about the mechanisms governing neonatal growth and maturation of organs. Here we demonstrate that calcineurin/Nuclear Factor of Activated T cells (Cn/NFAT) signaling regulates neonatal pancreatic development in mouse and human islets. Inactivation of calcineurin b1 (Cnb1) in mouse islets impaired dense core granule biogenesis, decreased insulin secretion, and reduced cell proliferation and mass, culminating in lethal diabetes. Pancreatic β cells lacking Cnb1 failed to express genes revealed to be direct NFAT targets required for replication, insulin storage, and secretion. In contrast, glucokinase activation stimulated Cn-dependent expression of these genes. Calcineurin inhibitors, such as tacrolimus, used for human immunosuppression, induce diabetes. Tacrolimus exposure reduced Cn/NFAT-dependent expression of factors essential for insulin dense core granule formation and secretion and neonatal β cell proliferation, consistent with our genetic studies. Discovery of conserved pathways regulating β cell maturation and proliferation suggests new strategies for controlling β cell growth or replacement in human islet diseases.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
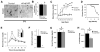

Comment in
-
Calcineurin/NFATc signaling: role in postnatal β cell development and diabetes mellitus.Dev Cell. 2012 Jul 17;23(1):7-8. doi: 10.1016/j.devcel.2012.06.009. Dev Cell. 2012. PMID: 22814597
References
-
- Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, Vanderbilt CA, Cobb MH. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–32525. - PubMed
-
- Arron JR, Winslow MM, Polleri A, Chang C-P, Neilson JR, Heit JJ, Kim SK, Francke U, Graef IA, Crabtree GR. NFAT dysregulation by increased dosage of DSCR1 and DYRK1a on chromosome 21. Nature. 2006;441:595–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

