Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study
- PMID: 22815429
- PMCID: PMC3400392
- DOI: 10.1136/bmj.e4565
Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study
Abstract
Objective: To evaluate the effect of different treatment strategies on enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome.
Design: Multicentre retrospective case-control study.
Setting: 23 hospitals in northern Germany.
Participants: 298 adults with enterohaemorrhagic E coli induced haemolytic uraemic syndrome.
Main outcome measures: Dialysis, seizures, mechanical ventilation, abdominal surgery owing to perforation of the bowel or bowel necrosis, and death.
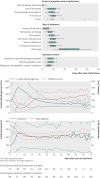
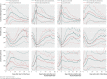
Results: 160 of the 298 patients (54%) temporarily required dialysis, with only three needing treatment long term. 37 patients (12%) had seizures, 54 (18%) required mechanical ventilation, and 12 (4%) died. No clear benefit was found from use of plasmapheresis or plasmapheresis with glucocorticoids. 67 of the patients were treated with eculizumab, a monoclonal antibody directed against the complement cascade. No short term benefit was detected that could be attributed to this treatment. 52 patients in one centre that used a strategy of aggressive treatment with combined antibiotics had fewer seizures (2% v 15%, P = 0.03), fewer deaths (0% v 5%, p = 0.029), required no abdominal surgery, and excreted E coli for a shorter duration.
Conclusions: Enterohaemorrhagic E coli induced haemolytic uraemic syndrome is a severe self limiting acute condition. Our findings question the benefit of eculizumab and of plasmapheresis with or without glucocorticoids. Patients with established haemolytic uraemic syndrome seemed to benefit from antibiotic treatment and this should be investigated in a controlled trial.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


Comment in
-
Treating Shiga toxin induced haemolytic uraemic syndrome.BMJ. 2012 Jul 19;345:e4598. doi: 10.1136/bmj.e4598. BMJ. 2012. PMID: 22815430 No abstract available.
-
Thrombotic microangiopathy: E. coli O104:H4 German outbreak: a missed opportunity.Nat Rev Nephrol. 2012 Oct;8(10):558-60. doi: 10.1038/nrneph.2012.194. Epub 2012 Sep 4. Nat Rev Nephrol. 2012. PMID: 22945489 No abstract available.
References
-
- Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, et al. Epidemic profile of shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011;365:1771-80. - PubMed
-
- Robert Koch Institut. Abschließende Darstellung und Bewertung der epidemiologischen Erkenntnisse im EHEC O104:H4 Ausbruch. 2011. [Final report on the epidemiology of the EHEC O104-H4 outbreak, Germany 2011.] www.rki.de/cln_117/nn_205760/DE/Content/InfAZ/E/EHEC/EHEC-Abschlussberic....
-
- Dundas S, Todd WT, Stewart AI, Murdoch PS, Chaudhuri AK, Hutchinson SJ. The central Scotland Escherichia coli O157:H7 outbreak: risk factors for the hemolytic uremic syndrome and death among hospitalized patients. Clin Infect Dis 2001;33:923-31. - PubMed
-
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005;365:1073-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
