Antiemetic treatment for acute gastroenteritis in children: an updated Cochrane systematic review with meta-analysis and mixed treatment comparison in a Bayesian framework
- PMID: 22815462
- PMCID: PMC3401831
- DOI: 10.1136/bmjopen-2011-000622
Antiemetic treatment for acute gastroenteritis in children: an updated Cochrane systematic review with meta-analysis and mixed treatment comparison in a Bayesian framework
Abstract
Objective: To assess the evidence for the safety and effectiveness of antiemetics on gastroenteritis-induced vomiting in children and adolescents.
Design: Systematic review.
Data sources: The Cochrane Central Register of Controlled Trials, MEDLINE and EMBASE searched from 1980 to March 2012.
Methods: Methods included comprehensive searches, data synthesis, meta-analysis and mixed treatment comparisons (MTC).
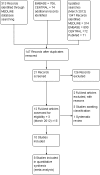
Review methods: Reference lists were checked, and missing or inconsistent data were sought from trial investigators. Randomised controlled trials comparing antiemetics in participants younger than 18 years and who were vomiting due to acute gastroenteritis. Four meta-analyses and three MTC were carried out.
Results: 10 trials (1479 participants) and five treatments were included: dexamethasone, dimenhydrinate, granisetron, metoclopramide and ondansetron. There was clear evidence that ondansetron (oral or intravenous) compared with placebo increased the proportion of patients with cessation of vomiting (orally administered) (RR 1.44, 95% CI 1.29 to 1.61), reduced the immediate hospital admission rate (orally administered) (RR 0.40, 95% CI 0.19 to 0.83) and the need for intravenous rehydration therapy (orally administered) (RR 0.41, 95% CI 0.29 to 0.59). No significant difference was noted in the revisit rates, but ondansetron was associated with an increase in episodes of diarrhoea. There was no evidence for the use of dexamethasone or metoclopramide and limited evidence that dimenhydrinate or granisetron increased the cessation of vomiting. The MTC analysis suggested that ondansetron was the most likely treatment to stop the child vomiting. Nine studies were carried out in secondary care and one in primary care.
Conclusions: This systematic review used a method novel to this clinical area and found clear evidence that ondansetron was the most likely treatment to allow oral rehydration therapy to commence. Given the significance of these results, the authors urge healthcare policy makers to consider the wider use of ondansetron in secondary care. Furthermore, randomised controlled trials are needed to investigate the effectiveness of antiemetic treatment in primary care (including ambulatory care interventions).
Conflict of interest statement
Figures




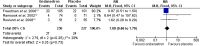
References
-
- OPCS Morbidity Statistics from General Practice. Forth National Study 1991–1992. London UK: HMSO, 1993
-
- American Academy of Pediatrics (AAP) Practice parameter: the management of acute gastroenteritis in young children. American academy of Pediatics, Provisional committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 1996;97:424–35 - PubMed
-
- National Collaborating Centre for Women's and Children's Health Diarrhoea and Vomiting Diagnosis, Assessment and Management in Children Younger than 5 years. London: RCOG Press, 2009 - PubMed
-
- Khanna R, Lakhanpaul M, Burman-Roy S, et al. Diarrhoea and vomiting caused by gastroenteritis in children under 5 years: summary of NICE guidance. BMJ 2009;25:1009–12 - PubMed
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
