The binding site of the V-ATPase inhibitor apicularen is in the vicinity of those for bafilomycin and archazolid
- PMID: 22815478
- PMCID: PMC3442520
- DOI: 10.1074/jbc.M112.372169
The binding site of the V-ATPase inhibitor apicularen is in the vicinity of those for bafilomycin and archazolid
Abstract
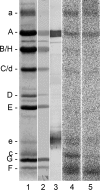
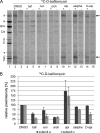
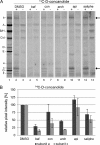
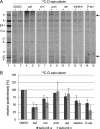
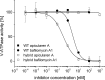
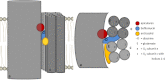
The investigation of V-ATPases as potential therapeutic drug targets and hence of their specific inhibitors is a promising approach in osteoporosis and cancer treatment because the occurrence of these diseases is interrelated to the function of the V-ATPase. Apicularen belongs to the novel inhibitor family of the benzolactone enamides, which are highly potent but feature the unique characteristic of not inhibiting V-ATPases from fungal sources. In this study we specify, for the first time, the binding site of apicularen within the membrane spanning V(O) complex. By photoaffinity labeling using derivatives of apicularen and of the plecomacrolides bafilomycin and concanamycin, each coupled to (14)C-labeled 4-(3-trifluoromethyldiazirin-3-yl)benzoic acid, we verified that apicularen binds at the interface of the V(O) subunits a and c. The binding site is in the vicinity to those of the plecomacrolides and of the archazolids, a third family of V-ATPase inhibitors. Expression of subunit c homologues from Homo sapiens and Manduca sexta, both species sensitive to benzolactone enamides, in a Saccharomyces cerevisiae strain lacking the corresponding intrinsic gene did not transfer this sensitivity to yeast. Therefore, the binding site of benzolactone enamides cannot be formed exclusively by subunit c. Apparently, subunit a substantially contributes to the binding of the benzolactone enamides.
Figures




Similar articles
-
Archazolid and apicularen: novel specific V-ATPase inhibitors.BMC Biochem. 2005 Aug 4;6:13. doi: 10.1186/1471-2091-6-13. BMC Biochem. 2005. PMID: 16080788 Free PMC article.
-
Inhibitors of V-ATPases: old and new players.J Exp Biol. 2009 Feb;212(Pt 3):341-6. doi: 10.1242/jeb.024067. J Exp Biol. 2009. PMID: 19151208 Review.
-
Archazolid A binds to the equatorial region of the c-ring of the vacuolar H+-ATPase.J Biol Chem. 2010 Dec 3;285(49):38304-14. doi: 10.1074/jbc.M110.137539. Epub 2010 Sep 30. J Biol Chem. 2010. PMID: 20884613 Free PMC article.
-
EPR Studies of V-ATPase with Spin-Labeled Inhibitors DCC and Archazolid: Interaction Dynamics with Proton Translocating Subunit c.ChemMedChem. 2016 Feb 17;11(4):420-8. doi: 10.1002/cmdc.201500500. Epub 2015 Dec 10. ChemMedChem. 2016. PMID: 26662886
-
V-ATPase inhibitors and implication in cancer treatment.Cancer Treat Rev. 2009 Dec;35(8):707-13. doi: 10.1016/j.ctrv.2009.08.003. Epub 2009 Sep 15. Cancer Treat Rev. 2009. PMID: 19758758 Review.
Cited by
-
Pharmacologic inhibition of vacuolar H+ ATPase reduces physiologic and oncogenic Notch signaling.Mol Oncol. 2014 Mar;8(2):207-20. doi: 10.1016/j.molonc.2013.11.002. Epub 2013 Nov 18. Mol Oncol. 2014. PMID: 24309677 Free PMC article.
-
PA1b inhibitor binding to subunits c and e of the vacuolar ATPase reveals its insecticidal mechanism.J Biol Chem. 2014 Jun 6;289(23):16399-408. doi: 10.1074/jbc.M113.541250. Epub 2014 May 2. J Biol Chem. 2014. PMID: 24795045 Free PMC article.
-
V-ATPases and osteoclasts: ambiguous future of V-ATPases inhibitors in osteoporosis.Theranostics. 2018 Oct 26;8(19):5379-5399. doi: 10.7150/thno.28391. eCollection 2018. Theranostics. 2018. PMID: 30555553 Free PMC article. Review.
-
A cell-based assay for CD63-containing extracellular vesicles.PLoS One. 2019 Jul 24;14(7):e0220007. doi: 10.1371/journal.pone.0220007. eCollection 2019. PLoS One. 2019. PMID: 31339911 Free PMC article.
-
Recent Insights into the Structure, Regulation, and Function of the V-ATPases.Trends Biochem Sci. 2015 Oct;40(10):611-622. doi: 10.1016/j.tibs.2015.08.005. Trends Biochem Sci. 2015. PMID: 26410601 Free PMC article. Review.
References
-
- Beyenbach K. W., Wieczorek H. (2006) The V-type H+ ATPase. Molecular structure and function, physiological roles, and regulation. J. Exp. Biol. 209, 577–589 - PubMed
-
- Forgac M. (2007) Vacuolar ATPases. Rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 - PubMed
-
- Muench S. P., Huss M., Song C. F., Phillips C., Wieczorek H., Trinick J., Harrison M. A. (2009) Cryoelectron microscopy of the vacuolar ATPase motor reveals its mechanical and regulatory complexity. J. Mol. Biol. 386, 989–999 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

