Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis
- PMID: 22815493
- PMCID: PMC6621291
- DOI: 10.1523/JNEUROSCI.0482-12.2012
Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis
Abstract
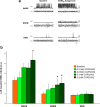
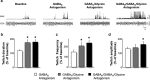
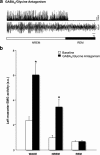
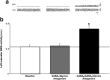
During REM sleep the CNS is intensely active, but the skeletal motor system is paradoxically forced into a state of muscle paralysis. The mechanisms that trigger REM sleep paralysis are a matter of intense debate. Two competing theories argue that it is caused by either active inhibition or reduced excitation of somatic motoneuron activity. Here, we identify the transmitter and receptor mechanisms that function to silence skeletal muscles during REM sleep. We used behavioral, electrophysiological, receptor pharmacology and neuroanatomical approaches to determine how trigeminal motoneurons and masseter muscles are switched off during REM sleep in rats. We show that a powerful GABA and glycine drive triggers REM paralysis by switching off motoneuron activity. This drive inhibits motoneurons by targeting both metabotropic GABA(B) and ionotropic GABA(A)/glycine receptors. REM paralysis is only reversed when motoneurons are cut off from GABA(B), GABA(A) and glycine receptor-mediated inhibition. Neither metabotropic nor ionotropic receptor mechanisms alone are sufficient for generating REM paralysis. These results demonstrate that multiple receptor mechanisms trigger REM sleep paralysis. Breakdown in normal REM inhibition may underlie common sleep motor pathologies such as REM sleep behavior disorder.
Figures





References
-
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–624. - PubMed
-
- Balasubramanian S, Teissére JA, Raju DV, Hall RA. Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J Biol Chem. 2004;279:18840–18850. - PubMed
-
- Barilà B, Cupello A, Robello M. GABA(B) receptor activation protects GABA(A) receptor from cyclic AMP-dependent down-regulation in rat cerebellar granule cells. Neuroscience. 1999;93:1077–1082. - PubMed
-
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. - PubMed
-
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
