Visuomotor functional network topology predicts upcoming tasks
- PMID: 22815510
- PMCID: PMC6621299
- DOI: 10.1523/JNEUROSCI.1604-12.2012
Visuomotor functional network topology predicts upcoming tasks
Abstract
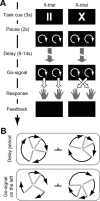
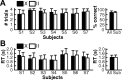
It is a vital ability of humans to flexibly adapt their behavior to different environmental situations. Constantly, the rules for our sensory-to-motor mappings need to be adapted to the current task demands. For example, the same sensory input might require two different motor responses depending on the actual situation. How does the brain prepare for such different responses? It has been suggested that the functional connections within cortex are biased according to the present rule to guide the flow of information in accordance with the required sensory-to-motor mapping. Here, we investigated with fMRI whether task settings might indeed change the functional connectivity structure in a large-scale brain network. Subjects performed a visuomotor response task that required an interaction between visual and motor cortex: either within each hemisphere or across the two hemispheres of the brain depending on the task condition. A multivariate analysis on the functional connectivity graph of a cortical visuomotor network revealed that the functional integration, i.e., the connectivity structure, is altered according to the task condition already during a preparatory period before the visual cue and the actual movement. Our results show that the topology of connection weights within a single network changes according to and thus predicts the upcoming task. This suggests that the human brain prepares to respond in different conditions by altering its large scale functional connectivity structure even before an action is required.
Figures





Similar articles
-
Dynamic Reconfiguration of Visuomotor-Related Functional Connectivity Networks.J Neurosci. 2017 Jan 25;37(4):839-853. doi: 10.1523/JNEUROSCI.1672-16.2016. J Neurosci. 2017. PMID: 28123020 Free PMC article.
-
Multiple movement representations in the human brain: an event-related fMRI study.J Cogn Neurosci. 2002 Jul 1;14(5):769-84. doi: 10.1162/08989290260138663. J Cogn Neurosci. 2002. PMID: 12167261
-
Large-scale Functional Integration, Rather than Functional Dissociation along Dorsal and Ventral Streams, Underlies Visual Perception and Action.J Cogn Neurosci. 2020 May;32(5):847-861. doi: 10.1162/jocn_a_01527. Epub 2020 Jan 14. J Cogn Neurosci. 2020. PMID: 31933430
-
Distributed motor processing in cerebral cortex.Curr Opin Neurobiol. 1994 Dec;4(6):840-6. doi: 10.1016/0959-4388(94)90132-5. Curr Opin Neurobiol. 1994. PMID: 7888767 Review.
-
Interactions between areas of the cortical grasping network.Curr Opin Neurobiol. 2011 Aug;21(4):565-70. doi: 10.1016/j.conb.2011.05.021. Epub 2011 Jun 21. Curr Opin Neurobiol. 2011. PMID: 21696944 Free PMC article. Review.
Cited by
-
Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns.Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):8762-7. doi: 10.1073/pnas.1501242112. Epub 2015 Jun 29. Proc Natl Acad Sci U S A. 2015. PMID: 26124112 Free PMC article.
-
Classification of self-driven mental tasks from whole-brain activity patterns.PLoS One. 2014 May 13;9(5):e97296. doi: 10.1371/journal.pone.0097296. eCollection 2014. PLoS One. 2014. PMID: 24824899 Free PMC article.
-
Sensory Integration: Cross-Modal Communication Between the Olfactory and Visual Systems in Zebrafish.Chem Senses. 2019 Jul 17;44(6):351-356. doi: 10.1093/chemse/bjz022. Chem Senses. 2019. PMID: 31066902 Free PMC article. Review.
-
Improved whole-brain reconfiguration efficiency reveals mechanisms of speech rehabilitation in cleft lip and palate patients: an fMRI study.Front Aging Neurosci. 2025 Mar 4;17:1536658. doi: 10.3389/fnagi.2025.1536658. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40103926 Free PMC article.
-
Higher Intelligence Is Associated with Less Task-Related Brain Network Reconfiguration.J Neurosci. 2016 Aug 17;36(33):8551-61. doi: 10.1523/JNEUROSCI.0358-16.2016. J Neurosci. 2016. PMID: 27535904 Free PMC article.
References
-
- Alemán-Gómez Y, Melie-García L, Valdés-Hernandez P. IBASPM: toolbox for automatic parcellation of brain structures. Paper presented at 12th annual meeting of the Organization for Human Brain Mapping; Florence, Italy. 2006.
-
- Bishop CM. Pattern recognition and machine learning (information science and statistics) New York: Springer; 2006.
-
- Bode S, Haynes JD. Decoding sequential stages of task preparation in the human brain. Neuroimage. 2009;45:606–613. - PubMed
-
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
