Expression of Ccl11 associates with immune response modulation and protection against neuroinflammation in rats
- PMID: 22815714
- PMCID: PMC3397980
- DOI: 10.1371/journal.pone.0039794
Expression of Ccl11 associates with immune response modulation and protection against neuroinflammation in rats
Abstract
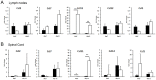
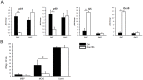
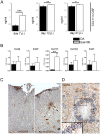
Multiple sclerosis (MS) is a polygenic disease characterized by inflammation and demyelination in the central nervous system (CNS), which can be modeled in experimental autoimmune encephalomyelitis (EAE). The Eae18b locus on rat chromosome 10 has previously been linked to regulation of beta-chemokine expression and severity of EAE. Moreover, the homologous chemokine cluster in humans showed evidence of association with susceptibility to MS. We here established a congenic rat strain with Eae18b locus containing a chemokine cluster (Ccl2, Ccl7, Ccl11, Ccl12 and Ccl1) from the EAE- resistant PVG rat strain on the susceptible DA background and utilized myelin oligodendrocyte glycoprotein (MOG)-induced EAE to characterize the mechanisms underlying the genetic regulation. Congenic rats developed a milder disease compared to the susceptible DA strain, and this was reflected in decreased demyelination and in reduced recruitment of inflammatory cells to the brain. The congenic strain also showed significantly increased Ccl11 mRNA expression in draining lymph nodes and spinal cord after EAE induction. In the lymph nodes, macrophages were the main producers of CCL11, whereas macrophages and lymphocytes expressed the main CCL11 receptor, namely CCR3. Accordingly, the congenic strain also showed significantly increased Ccr3 mRNA expression in lymph nodes. In the CNS, the main producers of CCL11 were neurons, whereas CCR3 was detected on neurons and CSF producing ependymal cells. This corresponded to increased levels of CCL11 protein in the cerebrospinal fluid of the congenic rats. Increased intrathecal production of CCL11 in congenic rats was accompanied by a tighter blood brain barrier, reflected by more occludin(+) blood vessels. In addition, the congenic strain showed a reduced antigen specific response and a predominant anti-inflammatory Th2 phenotype. These results indicate novel mechanisms in the genetic regulation of neuroinflammation.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

