Dopamine D4 receptor activation increases hippocampal gamma oscillations by enhancing synchronization of fast-spiking interneurons
- PMID: 22815864
- PMCID: PMC3398948
- DOI: 10.1371/journal.pone.0040906
Dopamine D4 receptor activation increases hippocampal gamma oscillations by enhancing synchronization of fast-spiking interneurons
Abstract
Background: Gamma oscillations are electric activity patterns of the mammalian brain hypothesized to serve attention, sensory perception, working memory and memory encoding. They are disrupted or altered in schizophrenic patients with associated cognitive deficits, which persist in spite of treatment with antipsychotics. Because cognitive symptoms are a core feature of schizophrenia it is relevant to explore signaling pathways that potentially regulate gamma oscillations. Dopamine has been reported to decrease gamma oscillation power via D1-like receptors. Based on the expression pattern of D4 receptors (D4R) in hippocampus, and pharmacological effects of D4R ligands in animals, we hypothesize that they are in a position to regulate gamma oscillations as well.
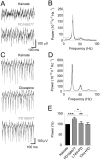
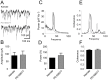
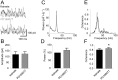
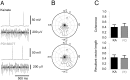
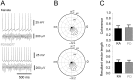
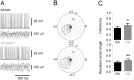
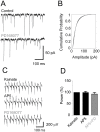
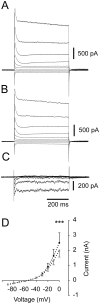
Methodology/principal findings: To address this hypothesis we use rat hippocampal slices and kainate-induced gamma oscillations. Local field potential recordings as well as intracellular recordings of pyramidal cells, fast-spiking and non-fast-spiking interneurons were carried out. We show that D4R activation with the selective ligand PD168077 increases gamma oscillation power, which can be blocked by the D4R-specific antagonist L745,870 as well as by the antipsychotic drug Clozapine. Pyramidal cells did not exhibit changes in excitatory or inhibitory synaptic current amplitudes, but inhibitory currents became more coherent with the oscillations after application of PD168077. Fast-spiking, but not non-fast spiking, interneurons, increase their action potential phase-coupling and coherence with regard to ongoing gamma oscillations in response to D4R activation. Among several possible mechanisms we found that the NMDA receptor antagonist AP5 also blocks the D4R mediated increase in gamma oscillation power.
Conclusions/significance: We conclude that D4R activation affects fast-spiking interneuron synchronization and thereby increases gamma power by an NMDA receptor-dependent mechanism. This suggests that converging deficits on fast-spiking interneurons may lead to decreased network function and thus aberrant gamma oscillations and cognitive decline in schizophrenia.
Conflict of interest statement
Figures
References
-
- Jensen O, Kaiser J, Lachaux J-P. Human gamma-frequency oscillations associated with attention and memory. TiNS. 2007;30:317–324. - PubMed
-
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, et al. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. - PubMed
-
- Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

