Increased autophagy in placentas of intrauterine growth-restricted pregnancies
- PMID: 22815878
- PMCID: PMC3397998
- DOI: 10.1371/journal.pone.0040957
Increased autophagy in placentas of intrauterine growth-restricted pregnancies
Abstract
Background: Unexplained intrauterine growth restriction (IUGR) may be a consequence of placental insufficiency; however, its etiology is not fully understood. We surmised that defective placentation in IUGR dysregulates cellular bioenergic homeostasis, leading to increased autophagy in the villous trophoblast. The aims of this work were (1) to compare the differences in autophagy, p53 expression, and apoptosis between placentas of women with normal or IUGR pregnancies; (2) to study the effects of hypoxia and the role of p53 in regulating trophoblast autophagy; and (3) to investigate the relationship between autophagy and apoptosis in hypoxic trophoblasts.
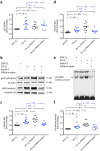
Methodology/principal findings: Compared with normal pregnant women, women with IUGR had higher placental levels of autophagy-related proteins LC3B-II, beclin-1, and damage-regulated autophagy modulator (DRAM), with increased p53 and caspase-cleaved cytokeratin 18 (M30). Furthermore, cytotrophoblasts cultured under hypoxia (2% oxygen) in the presence or absence of nutlin-3 (a p53 activity stimulator) had higher levels of LC3B-II, DRAM, and M30 proteins and increased Bax mRNA expression compared with controls cultured under standard conditions. In contrast, administration of pifithrin-α (a p53 activity inhibitor) during hypoxia resulted in protein levels that were similar to those of the control groups. Moreover, cytotrophoblasts transfected with LC3B, beclin-1, or DRAM siRNA had higher levels of M30 compared with the controls under hypoxia. However, transfection with Bcl-2 or Bax siRNA did not cause any significant change in the levels of LC3B-II in hypoxic cytotrophoblasts.
Conclusions/significance: Together, these results suggest that there is a crosstalk between autophagy and apoptosis in IUGR and that p53 plays a pivotal and complex role in regulating trophoblast cell turnover in response to hypoxic stress.
Conflict of interest statement
Figures









References
-
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. - PubMed
-
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. DevCell. 2004;6:463–477. - PubMed
-
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

