Ovarian cancer gene therapy using HPV-16 pseudovirion carrying the HSV-tk gene
- PMID: 22815887
- PMCID: PMC3398866
- DOI: 10.1371/journal.pone.0040983
Ovarian cancer gene therapy using HPV-16 pseudovirion carrying the HSV-tk gene
Abstract
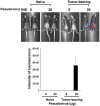
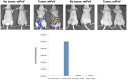
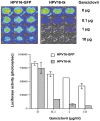
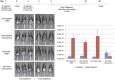
Ovarian cancer is the leading cause of death from all gynecological cancers and conventional therapies such as surgery, chemotherapy, and radiotherapy usually fail to control advanced stages of the disease. Thus, there is an urgent need for alternative and innovative therapeutic options. We reason that cancer gene therapy using a vector capable of specifically delivering an enzyme-encoding gene to ovarian cancer cells will allow the cancer cell to metabolize a harmless prodrug into a potent cytotoxin, which will lead to therapeutic effects. In the current study, we explore the use of a human papillomavirus (HPV) pseudovirion to deliver a herpes simplex virus thymidine kinase (HSV-tk) gene to ovarian tumor cells. We found that the HPV-16 pseudovirion was able to preferentially infect murine and human ovarian tumor cells when administered intraperitoneally. Furthermore, intraperitoneal injection of HPV-16 pseudovirions carrying the HSV-tk gene followed by treatment with ganciclovir led to significant therapeutic anti-tumor effects in murine ovarian cancer-bearing mice. Our data suggest that HPV pseudovirion may serve as a potential delivery vehicle for ovarian cancer gene therapy.
Conflict of interest statement
Figures

Similar articles
-
Systemic administration of radiation-potentiated anti-angiogenic gene therapy against primary and metastatic cancer based on transcriptionally controlled HSV-TK.Cancer Biol Ther. 2009 Mar;8(5):424-32. doi: 10.4161/cbt.8.5.7589. Epub 2009 Mar 19. Cancer Biol Ther. 2009. PMID: 19276657
-
Adenoviral-mediated delivery of the herpes simplex virus thymidine kinase gene selectively sensitizes human ovarian carcinoma cells to ganciclovir.Clin Cancer Res. 1995 Dec;1(12):1571-80. Clin Cancer Res. 1995. PMID: 9815958
-
Experimental study of the RV-HSV-TK/GCV suicide gene therapy system in gastric cancer.Cancer Biother Radiopharm. 2007 Dec;22(6):755-61. doi: 10.1089/cbr.2007.346. Cancer Biother Radiopharm. 2007. PMID: 18158766
-
Characteristics of ovarian cancer cells transduced by the bicistronic retroviral vector containing GM-CSF and HSV-TK genes.Chin Med J (Engl). 2001 Feb;114(2):147-51. Chin Med J (Engl). 2001. PMID: 11780195
-
Suicide gene strategies applied in ovarian cancer studies.Cancer Gene Ther. 2023 Jun;30(6):812-821. doi: 10.1038/s41417-023-00590-6. Epub 2023 Jan 30. Cancer Gene Ther. 2023. PMID: 36717737 Review.
Cited by
-
Effects of HPV Pseudotype Virus in Cutting E6 Gene Selectively in SiHa Cells.Curr Med Sci. 2018 Apr;38(2):212-221. doi: 10.1007/s11596-018-1868-3. Epub 2018 Apr 30. Curr Med Sci. 2018. PMID: 30074178
-
Immunogenic Human Papillomavirus Pseudovirus-Mediated Suicide-Gene Therapy for Bladder Cancer.Int J Mol Sci. 2016 Jul 14;17(7):1125. doi: 10.3390/ijms17071125. Int J Mol Sci. 2016. PMID: 27428950 Free PMC article.
-
Recent Progress in Gene Therapy for Ovarian Cancer.Int J Mol Sci. 2018 Jun 30;19(7):1930. doi: 10.3390/ijms19071930. Int J Mol Sci. 2018. PMID: 29966369 Free PMC article. Review.
-
Harnessing Human Papillomavirus' Natural Tropism to Target Tumors.Viruses. 2022 Jul 28;14(8):1656. doi: 10.3390/v14081656. Viruses. 2022. PMID: 36016277 Free PMC article. Review.
-
Human papillomavirus capsids preferentially bind and infect tumor cells.Int J Cancer. 2016 Feb 15;138(4):901-11. doi: 10.1002/ijc.29823. Epub 2015 Oct 27. Int J Cancer. 2016. PMID: 26317490 Free PMC article.
References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: a cancer journal for clinicians. 2000;50:7–33. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer statistics, 2006. CA: a cancer journal for clinicians. 2006;56:106–130. - PubMed
-
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer treatment and research. 2002;107:99–118. - PubMed
-
- Altaner C. Prodrug cancer gene therapy. Cancer letters. 2008;270:191–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

