Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis
- PMID: 22815907
- PMCID: PMC3398879
- DOI: 10.1371/journal.pone.0041047
Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis
Abstract
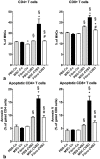
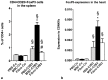
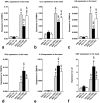
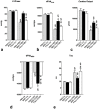
Systemic application of mesenchymal stromal cells (MSCs) in inflammatory cardiomyopathy exerts cardiobeneficial effects. The mode of action is unclear since a sufficient and long-acting cardiac homing of MSCs is unlikely. We therefore investigated the regulation of the immune response in coxsackievirus B3 (CVB3)-induced acute myocarditis after intravenous application of MSCs. Wildtype mice were infected with CVB3 and treated with either PBS, human MSCs or human cardiac fibroblasts intravenously 1 day after infection. Seven days after infection, MSCs could be detected in the spleen, heart, pancreas, liver, lung and kidney, whereby the highest presence was observed in the lung. MSCs increased significantly the myocardial expression of HGF and decreased the expression of the proinflammatory cytokines TNFα, IL1β and IL6 as well as the severity of myocarditis and ameliorated the left ventricular dysfunction measured by conductance catheter. MSCs upregulated the production of IFNγ in CD4+ and CD8+ cells, the number of IL10-producing regulatory T cells and the apoptosis rate of T cells in the spleen. An increased number of CD4+CD25+FoxP3 could be found in the spleen as well as in the circulation. In contrast, application of human cardiac fibroblasts had no effect on the severity of myocarditis and the systemic immune response observed after MSCs-administration. In conclusion, modulation of the immune response in extracardiac organs is associated with cardiobeneficial effects in experimental inflammatory cardiomyopathy after systemic application of MSCs.
Conflict of interest statement
Figures







Similar articles
-
Mesenchymal Stromal Cells Modulate Monocytes Trafficking in Coxsackievirus B3-Induced Myocarditis.Stem Cells Transl Med. 2017 Apr;6(4):1249-1261. doi: 10.1002/sctm.16-0353. Epub 2017 Jan 3. Stem Cells Transl Med. 2017. PMID: 28186704 Free PMC article.
-
Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis.Eur Heart J. 2011 Sep;32(17):2168-78. doi: 10.1093/eurheartj/ehq467. Epub 2010 Dec 22. Eur Heart J. 2011. PMID: 21183501 Free PMC article.
-
TNF-α/IL-1β-licensed mesenchymal stromal cells promote corneal allograft survival via myeloid cell-mediated induction of Foxp3+ regulatory T cells in the lung.FASEB J. 2019 Aug;33(8):9404-9421. doi: 10.1096/fj.201900047R. Epub 2019 May 20. FASEB J. 2019. PMID: 31108041
-
Mesenchymal stromal cells: a promising cell source for the treatment of inflammatory cardiomyopathy.Curr Pharm Des. 2011 Oct;17(30):3295-307. doi: 10.2174/138161211797904136. Curr Pharm Des. 2011. PMID: 21919878 Review.
-
The Roles of Cardiac Fibroblasts and Endothelial Cells in Myocarditis.Front Cardiovasc Med. 2022 Apr 7;9:882027. doi: 10.3389/fcvm.2022.882027. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35463742 Free PMC article. Review.
Cited by
-
Inhibition of LPS-Induced Inflammatory Response of Oral Mesenchymal Stem Cells in the Presence of Galectin-3.Biomedicines. 2023 May 24;11(6):1519. doi: 10.3390/biomedicines11061519. Biomedicines. 2023. PMID: 37371614 Free PMC article.
-
Cardiac migration of endogenous mesenchymal stromal cells in patients with inflammatory cardiomyopathy.Mediators Inflamm. 2015;2015:308185. doi: 10.1155/2015/308185. Epub 2015 Feb 28. Mediators Inflamm. 2015. PMID: 25814787 Free PMC article.
-
A Toolbox of Potential Immune-Related Therapies for Inflammatory Cardiomyopathy.J Cardiovasc Transl Res. 2021 Feb;14(1):75-87. doi: 10.1007/s12265-020-10025-4. Epub 2020 May 21. J Cardiovasc Transl Res. 2021. PMID: 32440911 Free PMC article. Review.
-
Q&A: Mesenchymal stem cells - where do they come from and is it important?BMC Biol. 2015 Nov 23;13:99. doi: 10.1186/s12915-015-0212-7. BMC Biol. 2015. PMID: 26596888 Free PMC article.
-
Therapeutic Effects of Lyophilized Conditioned-Medium Derived from Corneal Mesenchymal Stromal Cells on Corneal Epithelial Wound Healing.Curr Eye Res. 2020 Dec;45(12):1490-1496. doi: 10.1080/02713683.2020.1762227. Epub 2020 May 14. Curr Eye Res. 2020. PMID: 32338541 Free PMC article.
References
-
- Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol. 1991;68:1388–1392. - PubMed
-
- Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. - PubMed
-
- Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. - PubMed
-
- Kühl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. - PubMed
-
- Yajima T, Knowlton KU. Viral myocarditis: from the perspective of the virus. Circulation. 2009;119:2615–2624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

