Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities
- PMID: 22815931
- PMCID: PMC3398889
- DOI: 10.1371/journal.pone.0041105
Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities
Erratum in
- PLoS One. 2012;7(10). doi:10.1371/annotation/f6ebe3d3-ef7c-42ce-86fe-d5a661d7f67f
Abstract
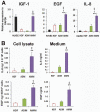
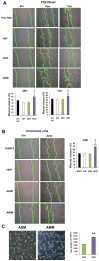
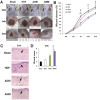
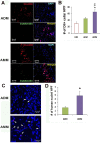
Although human amniotic mesenchymal stem cells (AMMs) have been recognised as a promising stem cell resource, their therapeutic potential for wound healing has not been widely investigated. In this study, we evaluated the therapeutic potential of AMMs using a diabetic mouse wound model. Quantitative real-time PCR and ELISA results revealed that the angiogenic factors, IGF-1, EGF and IL-8 were markedly upregulated in AMMs when compared with adipose-derived mesenchymal stem cells (ADMs) and dermal fibroblasts. In vitro scratch wound assays also showed that AMM-derived conditioned media (CM) significantly accelerated wound closure. Diabetic mice were generated using streptozotocin and wounds were created by skin excision, followed by AMM transplantation. AMM transplantation significantly promoted wound healing and increased re-epithelialization and cellularity. Notably, transplanted AMMs exhibited high engraftment rates and expressed keratinocyte-specific proteins and cytokeratin in the wound area, indicating a direct contribution to cutaneous closure. Taken together, these data suggest that AMMs possess considerable therapeutic potential for chronic wounds through the secretion of angiogenic factors and enhanced engraftment/differentiation capabilities.
Conflict of interest statement
Figures


References
-
- Sumpio BE, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:689–708. - PubMed
-
- Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–494. - PubMed
-
- Campbell WB, Ponette D, Sugiono M. Long-term results following operation for diabetic foot problems: arterial disease confers a poor prognosis. Eur J Vasc Endovasc Surg. 2000;19:174–177. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

