Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression
- PMID: 22815945
- PMCID: PMC3399807
- DOI: 10.1371/journal.pone.0041147
Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression
Abstract
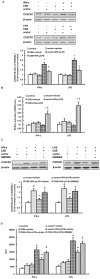
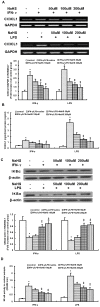
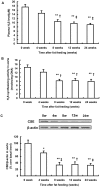
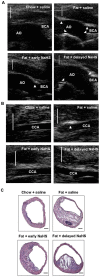
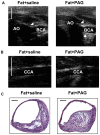
Hydrogen sulfide, as a novel gaseous mediator, has been suggested to play a key role in atherogenesis. However, the precise mechanisms by which H(2)S affects atherosclerosis remain unclear. Therefore, the present study aimed to investigate the potential role of H(2)S in atherosclerosis and the underlying mechanism with respect to chemokines (CCL2, CCL5 and CX3CL1) and chemokine receptors (CCR2, CCR5, and CX3CR1) in macrophages. Mouse macrophage cell line RAW 264.7 or mouse peritoneal macrophages were pre-incubated with saline or NaHS (50 µM, 100 µM, 200 µM), an H(2)S donor, and then stimulated with interferon-γ (IFN-γ) or lipopolysaccharide (LPS). It was found that NaHS dose-dependently inhibited IFN-γ or LPS-induced CX3CR1 and CX3CL1 expression, as well as CX3CR1-mediated chemotaxis in macrophages. Overexpression of cystathionine γ-lyase (CSE), an enzyme that catalyzes H(2)S biosynthesis resulted in a significant reduction in CX3CR1 and CX3CL1 expression as well as CX3CR1-mediated chemotaxis in stimulated macrophages. The inhibitory effect of H(2)S on CX3CR1 and CX3CL1 expression was mediated by modulation of proliferators-activated receptor-γ (PPAR-γ) and NF-κB pathway. Furthermore, male apoE(-/-) mice were fed a high-fat diet and then randomly given NaHS (1 mg/kg, i.p., daily) or DL-propargylglycine (PAG, 10 mg/kg, i.p., daily). NaHS significantly inhibited aortic CX3CR1 and CX3CL1 expression and impeded aortic plaque development. NaHS had a better anti-atherogenic benefit when it was applied at the early stage of atherosclerosis. However, inhibition of H(2)S formation by PAG increased aortic CX3CR1 and CX3CL1 expression and exacerbated the extent of atherosclerosis. In addition, H(2)S had minimal effect on the expression of CCL2, CCL5, CCR2 and CCR5 in vitro and in vivo. In conclusion, these data indicate that H(2)S hampers the progression of atherosclerosis in fat-fed apoE(-/-) mice and downregulates CX3CR1 and CX3CL1 expression on macrophages and in lesion plaques.
Conflict of interest statement
Figures






Similar articles
-
Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice.Eur J Pharmacol. 2012 Dec 15;697(1-3):106-16. doi: 10.1016/j.ejphar.2012.10.005. Epub 2012 Oct 16. Eur J Pharmacol. 2012. PMID: 23085268
-
Dysregulation of cystathionine γ-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage.Cell Signal. 2013 Nov;25(11):2255-62. doi: 10.1016/j.cellsig.2013.07.010. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872072
-
Human CD16+ monocytes promote a pro-atherosclerotic endothelial cell phenotype via CX3CR1-CX3CL1 interaction.Cardiovasc Res. 2021 May 25;117(6):1510-1522. doi: 10.1093/cvr/cvaa234. Cardiovasc Res. 2021. PMID: 32717023
-
An oxidized lipid-peroxisome proliferator-activated receptor gamma-chemokine pathway in the regulation of macrophage-vascular smooth muscle cell adhesion.Trends Cardiovasc Med. 2007 Nov;17(8):269-74. doi: 10.1016/j.tcm.2007.09.004. Trends Cardiovasc Med. 2007. PMID: 18021937 Free PMC article. Review.
-
CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration.J Neuroinflammation. 2010 Dec 2;7:87. doi: 10.1186/1742-2094-7-87. J Neuroinflammation. 2010. PMID: 21126357 Free PMC article. Review.
Cited by
-
Hydrogen sulfide as a vasculoprotective factor.Med Gas Res. 2013 Apr 29;3(1):9. doi: 10.1186/2045-9912-3-9. Med Gas Res. 2013. PMID: 23628084 Free PMC article.
-
Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease.Int J Mol Sci. 2022 Oct 13;23(20):12202. doi: 10.3390/ijms232012202. Int J Mol Sci. 2022. PMID: 36293058 Free PMC article. Review.
-
Hydrogen Sulfide and its Interaction with Other Players in Inflammation.Adv Exp Med Biol. 2021;1315:129-159. doi: 10.1007/978-981-16-0991-6_6. Adv Exp Med Biol. 2021. PMID: 34302691
-
Hydrogen Sulfide Attenuates Atherosclerosis in a Partially Ligated Carotid Artery Mouse model via Regulating Angiotensin Converting Enzyme 2 Expression.Front Physiol. 2017 Oct 10;8:782. doi: 10.3389/fphys.2017.00782. eCollection 2017. Front Physiol. 2017. PMID: 29066981 Free PMC article.
-
Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARα.Int J Mol Sci. 2016 Apr 29;17(5):635. doi: 10.3390/ijms17050635. Int J Mol Sci. 2016. PMID: 27136542 Free PMC article.
References
-
- Renga B. Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE). Inflamm Allergy Drug Targets. 2011;10:85–91. - PubMed
-
- Elsey DJ, Fowkes RC, Baxter GF. Regulation of cardiovascular cell function by hydrogen sulfide (H2S). Cell Biochem Funct. 2010;28:95–106. - PubMed
-
- Wang Y, Zhao X, Jin H, Wei H, Li W, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179. - PubMed
-
- Zhao ZZ, Wang Z, Li GH, WanG R, Tan JM, et al. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011;236:169–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

