Leishmania amazonensis fails to induce the release of reactive oxygen intermediates by CBA macrophages
- PMID: 22817661
- PMCID: PMC3532614
- DOI: 10.1111/j.1365-3024.2012.01384.x
Leishmania amazonensis fails to induce the release of reactive oxygen intermediates by CBA macrophages
Abstract
CBA mouse macrophages effectively control Leishmania major infection, yet are permissive to Leishmania amazonensis. It has been established that some Leishmania species are destroyed by reactive oxygen species (ROS). However, other species of Leishmania exhibit resistance to ROS or even down-modulate ROS production. We hypothesized that L. amazonensis-infected macrophages reduce ROS production soon after parasite-cell interaction. Employing a highly sensitive analysis technique based on chemiluminescence, the production of superoxide (O(·-)(2)) and hydrogen peroxide (H(2)O(2)) by L. major- or L. amazonensis-infected CBA macrophages were measured. L. major induces macrophages to release levels of (O(·-)(2)) 3·5 times higher than in uninfected cells. This (O(·-)(2)) production is partially dependent on NADPH oxidase (NOX) type 2. The level of accumulated H(2)O(2) is 20 times higher in L. major-than in L. amazonensis-infected cells. Furthermore, macrophages stimulated with L. amazonensis release amounts of ROS similar to uninfected cells. These findings support previous studies showing that CBA macrophages are effective in controlling L. major infection by a mechanism dependent on both (O(·-)(2)) production and H(2)O(2) generation. Furthermore, these data reinforce the notion that L. amazonensis survive inside CBA macrophages by reducing ROS production during the phagocytic process.
© 2012 Blackwell Publishing Ltd.
Figures

 production. Thioglycolate-elicited peritoneal macrophages were incubated with L.
major or L. amazonensis promastigotes at a 10:1 ratio at 37°C for 30 min in the presence of lucigenin (25 μ
production. Thioglycolate-elicited peritoneal macrophages were incubated with L.
major or L. amazonensis promastigotes at a 10:1 ratio at 37°C for 30 min in the presence of lucigenin (25 μ production was measured using lucigenin-based chemiluminescence (CL), expressed in photon counts. L. major promastigotes induce the release of significantly higher amounts of
production was measured using lucigenin-based chemiluminescence (CL), expressed in photon counts. L. major promastigotes induce the release of significantly higher amounts of  in comparison with L. amazonensis (n = 11, P<0·001, One-way
in comparison with L. amazonensis (n = 11, P<0·001, One-way  production (one representative experiment out of eight similar experiments) (b). NOX inhibition by apocynin cause partial reduction in lucigenin-based CL. L. major-infected cells were pretreated for 18 h with apocynin (500 μ
production (one representative experiment out of eight similar experiments) (b). NOX inhibition by apocynin cause partial reduction in lucigenin-based CL. L. major-infected cells were pretreated for 18 h with apocynin (500 μ production was detected at 37°C for 10 min and was partially reduced by apocynin. Results are expressed as the percentage of the number of photons emitted by apocynin-treated cells (ranging from 57·6 to 193·9 photons) in relation to untreated macrophages considered as 100% (ranging from 126·2 to 318·7 photons) (n = 4, P=0·02, Mann–Whitney U-test) (c).
production was detected at 37°C for 10 min and was partially reduced by apocynin. Results are expressed as the percentage of the number of photons emitted by apocynin-treated cells (ranging from 57·6 to 193·9 photons) in relation to untreated macrophages considered as 100% (ranging from 126·2 to 318·7 photons) (n = 4, P=0·02, Mann–Whitney U-test) (c).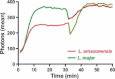
 production by macrophages sequentially stimulated with L. major or L. amazonensis promastigotes. Phagocytic assays were performed by incubating cells with L. major (green) or L. amazonensis (red) promastigotes for 30 min. Next, the parasite stimuli were switched, and the cells were incubated for a second 30-min period, with either L. amazonensis or L. major promastigote (10:1), respectively. These sequential stimulations were performed in the presence of lucigenin (25 μ
production by macrophages sequentially stimulated with L. major or L. amazonensis promastigotes. Phagocytic assays were performed by incubating cells with L. major (green) or L. amazonensis (red) promastigotes for 30 min. Next, the parasite stimuli were switched, and the cells were incubated for a second 30-min period, with either L. amazonensis or L. major promastigote (10:1), respectively. These sequential stimulations were performed in the presence of lucigenin (25 μ production was measured via lucigenin-based chemiluminescence emitted by cells. L. amazonensis did not revert the
production was measured via lucigenin-based chemiluminescence emitted by cells. L. amazonensis did not revert the  production induced by L. major in macrophage cultures, yet incubation with L.
major in cultures previously stimulated with L. amazonensis did revert relative low levels of
production induced by L. major in macrophage cultures, yet incubation with L.
major in cultures previously stimulated with L. amazonensis did revert relative low levels of  production (one representative experiment out of three similar experiments).
production (one representative experiment out of three similar experiments).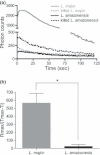
References
-
- Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99:17–23. - PubMed
-
- Russell DG, Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989;10:328–333. - PubMed
-
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967;46:19–28. - PubMed
-
- Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

