The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis
- PMID: 22817896
- PMCID: PMC3664196
- DOI: 10.1016/j.cell.2012.06.019
The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis
Abstract
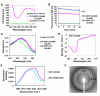
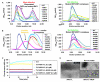
RIP1 and RIP3 kinases are central players in TNF-induced programmed necrosis. Here, we report that the RIP homotypic interaction motifs (RHIMs) of RIP1 and RIP3 mediate the assembly of heterodimeric filamentous structures. The fibrils exhibit classical characteristics of β-amyloids, as shown by Thioflavin T (ThT) and Congo red (CR) binding, circular dichroism, infrared spectroscopy, X-ray diffraction, and solid-state NMR. Structured amyloid cores are mapped in RIP1 and RIP3 that are flanked by regions of mobility. The endogenous RIP1/RIP3 complex isolated from necrotic cells binds ThT, is ultrastable, and has a fibrillar core structure, whereas necrosis is partially inhibited by ThT, CR, and another amyloid dye, HBX. Mutations in the RHIMs of RIP1 and RIP3 that are defective in the interaction compromise cluster formation, kinase activation, and programmed necrosis in vivo. The current study provides insight into the structural changes that occur when RIP kinases are triggered to execute different signaling outcomes and expands the realm of amyloids to complex formation and signaling.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Cell signalling: A necrosome build-up.Nat Rev Mol Cell Biol. 2012 Sep;13(9):540. doi: 10.1038/nrm3415. Epub 2012 Aug 1. Nat Rev Mol Cell Biol. 2012. PMID: 22850818 No abstract available.
-
Amyloids as kinase signalling platforms.Nat Rev Mol Cell Biol. 2023 Feb;24(2):85. doi: 10.1038/s41580-022-00553-z. Nat Rev Mol Cell Biol. 2023. PMID: 36266548 No abstract available.
References
-
- Avila J, Gómez de Barreda E, Engel T, Lucas JJ, Hernández F. Tau phosphorylation in hippocampus results in toxic gain-of-function. Biochem. Soc. Trans. 2010;38:977–980. - PubMed
-
- Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003;278:51613–51621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

