Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche
- PMID: 22817897
- PMCID: PMC4492542
- DOI: 10.1016/j.cell.2012.05.041
Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche
Abstract
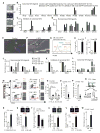
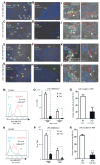
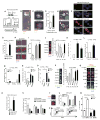
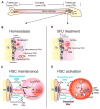
Wnt signaling is involved in self-renewal and maintenance of hematopoietic stem cells (HSCs); however, the particular role of noncanonical Wnt signaling in regulating HSCs in vivo is largely unknown. Here, we show Flamingo (Fmi) and Frizzled (Fz) 8, members of noncanonical Wnt signaling, both express in and functionally maintain quiescent long-term HSCs. Fmi regulates Fz8 distribution at the interface between HSCs and N-cadherin(+) osteoblasts (N-cad(+)OBs that enrich osteoprogenitors) in the niche. We further found that N-cad(+)OBs predominantly express noncanonical Wnt ligands and inhibitors of canonical Wnt signaling under homeostasis. Under stress, noncanonical Wnt signaling is attenuated and canonical Wnt signaling is enhanced in activation of HSCs. Mechanistically, noncanonical Wnt signaling mediated by Fz8 suppresses the Ca(2+)-NFAT- IFNγ pathway, directly or indirectly through the CDC42-CK1α complex and also antagonizes canonical Wnt signaling in HSCs. Taken together, our findings demonstrate that noncanonical Wnt signaling maintains quiescent long-term HSCs through Fmi and Fz8 interaction in the niche.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B, Zhang J, Haug J, Li L. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101:383–389. - PubMed
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

