Ten years of experience with biphasic insulin aspart 30: from drug development to the latest clinical findings
- PMID: 22818015
- PMCID: PMC3590411
- DOI: 10.2165/11635490-000000000-00000
Ten years of experience with biphasic insulin aspart 30: from drug development to the latest clinical findings
Abstract
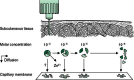
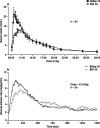
Biphasic insulin aspart 30 (BIAsp 30) includes 30% soluble rapid-acting insulin aspart (IAsp) along with an intermediate-acting 70% protaminated IAsp that provides coverage of prandial and basal insulin in a single injection. As BIAsp 30 has been available internationally for 10 years, this review provides a comprehensive overview of the discovery of BIAsp 30, its pharmacokinetic and pharmacodynamic profile, safety and efficacy outcomes from the clinical trial programme, 'real-life' clinical insights provided by observational study data, and cost effectiveness and quality-of-life information. These studies have demonstrated that BIAsp 30 once or twice daily is an appropriate option for insulin initiation. BIAsp 30 also provides a switch option in patients on biphasic human insulin (BHI). Switching from BHI to BIAsp 30 is associated with improved postprandial glucose (PPG) and reduced nocturnal and major hypoglycaemia, although daytime hypoglycaemia is higher with BIAsp 30. Intensification of BIAsp 30 can be achieved by increasing the number of daily doses up to three times daily with meals. Therefore, BIAsp 30 provides an intensification option for individuals who are not achieving control with basal insulin and would prefer the simplicity of a single biphasic insulin instead of progressing to a basal-bolus approach. BIAsp 30 has a simple dose-titration algorithm, which enables patients to effectively self-titrate their insulin dose. Cost-effectiveness analyses have demonstrated that BIAsp 30 is cost effective or dominant compared with BHI 30 or insulin glargine in a number of healthcare settings. In conclusion, BIAsp 30 offers a simple and flexible option for insulin initiation and intensification that provides coverage of both fasting and prandial glucose.
Figures











References
-
- DCCT The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289–98. - PubMed
-
- UK Prospective Diabetes Study Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. - PubMed
-
- Holman R.R., Paul S.K., Bethel M.A., et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. - PubMed
-
- Monnier L., Lapinski H., Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–5. - PubMed
-
- Leiter L.A., Ceriello A., Davidson J., International Prandial Glucose Regulation Study Group et al. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27(Suppl. B):S42–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

