Passing the baton: the HIF switch
- PMID: 22818162
- PMCID: PMC3433036
- DOI: 10.1016/j.tibs.2012.06.004
Passing the baton: the HIF switch
Abstract
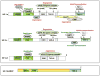
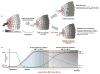
Hypoxia is an inadequate oxygen supply to tissues and cells, which can restrict their function. The hypoxic response is primarily mediated by the hypoxia-inducible transcription factors, HIF-1 and HIF-2, which have both overlapping and unique target genes. HIF target gene activation is highly context specific and is not a reliable indicator of which HIF-α isoform is active. For example, in some cell lines, the individual HIFs have specific temporal and functional roles: HIF-1 drives the initial response to hypoxia (<24h) and HIF-2 drives the chronic response (>24h). Here, we review the significance of the HIF switch and the relation between HIF-1 and HIF-2 under both physiological and pathophysiological conditions.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target.Angiogenesis. 2018 May;21(2):183-202. doi: 10.1007/s10456-018-9600-2. Epub 2018 Jan 27. Angiogenesis. 2018. PMID: 29383635 Free PMC article. Review.
-
Turn me on: regulating HIF transcriptional activity.Cell Death Differ. 2008 Apr;15(4):642-9. doi: 10.1038/sj.cdd.4402315. Epub 2008 Jan 18. Cell Death Differ. 2008. PMID: 18202699 Review.
-
Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells.Mol Cell Biol. 2006 May;26(9):3514-26. doi: 10.1128/MCB.26.9.3514-3526.2006. Mol Cell Biol. 2006. PMID: 16611993 Free PMC article.
-
Regulation of hypoxia-inducible gene expression after HIF activation.Exp Cell Res. 2017 Jul 15;356(2):182-186. doi: 10.1016/j.yexcr.2017.03.013. Epub 2017 Mar 9. Exp Cell Res. 2017. PMID: 28286304 Review.
-
Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response.Cell Signal. 2013 Sep;25(9):1895-903. doi: 10.1016/j.cellsig.2013.05.018. Epub 2013 May 21. Cell Signal. 2013. PMID: 23707522 Free PMC article. Review.
Cited by
-
ISCA2 inhibition decreases HIF and induces ferroptosis in clear cell renal carcinoma.Oncogene. 2022 Oct;41(42):4709-4723. doi: 10.1038/s41388-022-02460-1. Epub 2022 Sep 12. Oncogene. 2022. PMID: 36097192 Free PMC article.
-
Acute vs. chronic vs. intermittent hypoxia in breast Cancer: a review on its application in in vitro research.Mol Biol Rep. 2022 Nov;49(11):10961-10973. doi: 10.1007/s11033-022-07802-6. Epub 2022 Sep 3. Mol Biol Rep. 2022. PMID: 36057753 Free PMC article. Review.
-
Development of an oxygen-sensitive degradable peptide probe for the imaging of hypoxia-inducible factor-1-active regions in tumors.Mol Imaging Biol. 2013 Dec;15(6):713-21. doi: 10.1007/s11307-013-0647-6. Mol Imaging Biol. 2013. PMID: 23689986
-
Hypoxia-inducible factor-2α directly promotes BCRP expression and mediates the resistance of ovarian cancer stem cells to adriamycin.Mol Oncol. 2019 Feb;13(2):403-421. doi: 10.1002/1878-0261.12419. Epub 2019 Jan 14. Mol Oncol. 2019. PMID: 30536571 Free PMC article.
-
Exploiting metabolic vulnerabilities after anti-VEGF antibody therapy in ovarian cancer.iScience. 2023 Jan 19;26(2):106020. doi: 10.1016/j.isci.2023.106020. eCollection 2023 Feb 17. iScience. 2023. PMID: 36824283 Free PMC article.
References
-
- Pouyssegur J, et al. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. - PubMed
-
- Makino Y, et al. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277:32405–32408. - PubMed
-
- Holmquist-Mengelbier L, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

