Effect of processing and storage on red blood cell function in vivo
- PMID: 22818545
- PMCID: PMC3404625
- DOI: 10.1053/j.semperi.2012.04.005
Effect of processing and storage on red blood cell function in vivo
Abstract
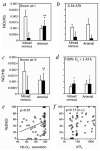
Red blood cell (RBC) transfusion is indicated to improve oxygen delivery to tissue, and for no other purpose. We have come to appreciate that donor RBCs are fundamentally altered during processing and storage in a manner that both impairs oxygen transport efficacy and introduces additional risk by perturbing both immune and coagulation systems. The protean biophysical and physiological changes in RBC function arising from storage are termed the "storage lesion;" many have been understood for some time; for example, we know that the oxygen affinity of stored blood rises during the storage period and that intracellular allosteric regulators, notably 2,3-bisphosphoglyceric acid and ATP, are depleted during storage. Our appreciation of other storage lesion features has emerged with improved understanding of coagulation, immune, and vascular signaling systems. Here, we review key features of the "storage lesion." Additionally, we call particular attention to the newly appreciated role of RBCs in regulating linkage between regional blood flow and regional O(2) consumption by regulating the bioavailability of key vasoactive mediators in plasma, and discuss how processing and storage disturb this key signaling function and impair transfusion efficacy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

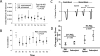
References
-
- Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008;36:2667–2674. - PubMed
-
- Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Azarow K, Holcomb JB. Risks associated with fresh whole blood and red blood cell transfusions in a combat support hospital. Crit Care Med. 2007;35:2576–2581. - PubMed
-
- Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: When is it not safe? Crit Care Med. 2003;31:S687–S697. - PubMed
-
- Marik PE, Sibbald WJ. Effect of stored blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. - PubMed
-
- Napolitano LM, Corwin HL. Efficacy of blood transfusion in the critically ill: Does age of blood make a difference? Crit Care Med. 2004;32:594–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

