Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma
- PMID: 22818878
- PMCID: PMC3438387
- DOI: 10.1016/j.ultrasmedbio.2012.04.015
Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma
Abstract
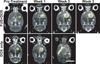
The blood-brain barrier (BBB) inhibits the entry of the majority of chemotherapeutic agents into the brain. Previous studies have illustrated the feasibility of drug delivery across the BBB using focused ultrasound (FUS) and microbubbles. Here, we investigated the effect of FUS-enhanced delivery of doxorubicin on survival in rats with and 9L gliosarcoma cells inoculated in the brain. Each rat received either: (1) no treatment (control; N = 11), (2) FUS only (N = 9), (3) IV liposomal doxorubicin (DOX only; N = 17), or (4) FUS with concurrent IV injections of liposomal doxorubicin (FUS+DOX; N = 20). Post-treatment by magnetic resonance imaging (MRI) showed that FUS+DOX reduced tumor growth compared with DOX only. Further, we observed a modest but significant increase in median survival time after a single treatment FUS+DOX treatment (p = 0.0007), whereas neither DOX nor FUS had any significant impact on survival on its own. These results suggest that combined ultrasound-mediated BBB disruption may significantly increase the antineoplastic efficacy of liposomal doxorubicin in the brain.
Copyright © 2012 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113:84–93. - PubMed
-
- Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. - PubMed
-
- Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33:95–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

