Bispecific targeting of EGFR and uPAR in a mouse model of head and neck squamous cell carcinoma
- PMID: 22818892
- PMCID: PMC3480964
- DOI: 10.1016/j.oraloncology.2012.06.002
Bispecific targeting of EGFR and uPAR in a mouse model of head and neck squamous cell carcinoma
Abstract
Objectives: To investigate the efficacy of the bispecific targeted toxin, dEGFATFKDEL, on head and neck carcinoma cell lines in vitro and in vivo.
Materials and methods: A deimmunized bispecific anti-cancer agent was constructed to simultaneously target both the overexpressed EGF receptor on carcinomas and the urokinase receptor (uPAR), that is found on the endothelial cells of the neovasculature within tumors. Flow cytometry assays were performed to determine the level of EGFR expressed on a variety of carcinoma lines. These lines were then tested in tritiated leucine incorporation assays to determine the efficacy of dEGFATFKDEL. Human vein endothelial primary cells were also tested to determine the effectiveness of the ATF portion of the molecule that binds uPAR. Furthermore, mouse studies were performed to determine whether dEGFATFKDEL was effective at inhibiting tumor growth in vivo.
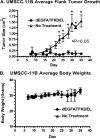
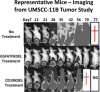
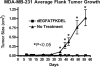
Results: UMSCC-11B and NA, two head and neck squamous cell carcinomas, highly expressed EGFR. Both the carcinoma lines and the human vein endothelial cells were inhibited at sub-nanomolar concentrations by dEGFATFKDEL. The tumor studies showed that the tumors treated with dEGFATFKDEL were significantly inhibited whereas the negative control and untreated tumors progressed. In a separate in vivo study involving another carcinoma line, MDA-MB-231, the effectiveness of dEGFATFKDEL was confirmed. No toxicity was seen at the doses used in either of these mouse studies.
Conclusions: This bispecific agent is effective in a mouse model of head and neck squamous cell carcinoma. Further study of this reagent for use in the treatment of carcinomas is warranted.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Bianchini C, Ciorba A, Pelucchi S, Piva R, Pastore A. Targeted therapy in head and neck cancer. Tumori. 2011 Mar-Apr;97(2):137–41. - PubMed
-
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of Resistance to Systemic Therapy in Patients with Breast Cancer. Adv Exp Med Biol. 2007;608:1–22. - PubMed
-
- Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Annals of Oncology. 2003;14:1346–1363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

