Engineered T cells for anti-cancer therapy
- PMID: 22818942
- PMCID: PMC3622551
- DOI: 10.1016/j.coi.2012.06.004
Engineered T cells for anti-cancer therapy
Abstract
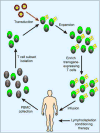
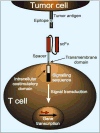
Recent advances enabling efficient delivery of transgenes to human T cells have created opportunities to address obstacles that previously hindered the application of T cell therapy to cancer. Modification of T cells with transgenes encoding TCRs or chimeric antigen receptors allows tumor specificity to be conferred on functionally distinct T cell subsets, and incorporation of costimulatory molecules or cytokines can enable engineered T cells to bypass local and systemic tolerance mechanisms. Clinical studies of genetically modified T cell therapy for cancer have shown notable success; however, these trials demonstrate that tumor therapy with engineered high avidity tumor-reactive T cells may be accompanied by significant on-target toxicity, necessitating careful selection of target antigens and development of strategies to eliminate transferred cells.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
References
-
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research an official journal of the American Association for Cancer Research. 2011;17:4550–4557. - PMC - PubMed
-
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanomaevaluation of intensive myeloablative chemoradiation preparative regimens. Journal of clinical oncology official journal of the American Society of Clinical Oncology. 2008;26:5233–5239. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

