Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins
- PMID: 22818981
- PMCID: PMC3418443
- DOI: 10.1016/j.biomaterials.2012.06.101
Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins
Abstract
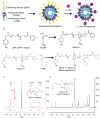
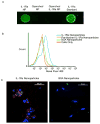
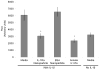
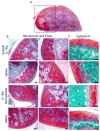
Intra-articular delivery of therapeutics to modulate osteoarthritis (OA) is challenging. Delivery of interleukin-1 receptor antagonist (IL-1Ra), the natural protein inhibitor of IL-1, to modulate IL-1-based inflammation through gene therapy or bolus protein injections has emerged as a promising therapy for OA. However, these approaches suffer from rapid clearance and reduced potency over time. Nano/microparticles represent a promising strategy for overcoming the shortcomings of intra-articular drug delivery. However, these delivery vehicles are limited for delivery of protein therapeutics due to their hydrophobic character, low drug loading efficiency, and harsh chemical conditions during particle processing. We designed a new block copolymer that assembles into submicron-scale particles and provides for covalently tethering proteins to the particle surface for controlled intra-articular protein delivery. This block copolymer self-assembles into 300 nm-diameter particles with a protein tethering moiety for surface covalent conjugation of IL-1Ra protein. This copolymer particle system efficiently bound IL-1Ra and maintained protein bioactivity in vitro. Furthermore, particle-tethered IL-1Ra bound specifically to target synoviocyte cells via surface IL-1 receptors. Importantly, IL-1Ra nanoparticles inhibited IL-1-mediated signaling to equivalent levels as soluble IL-1Ra. Finally, the ability of nanoparticles to retain IL-1Ra in the rat stifle joint was evaluated by in vivo imaging over 14 days. IL-1Ra-tethered nanoparticles significantly increased the retention time of IL-1Ra in the rat stifle joint over 14 days with enhanced IL-1Ra half-life (3.01 days) compared to that of soluble IL-1Ra (0.96 days) and without inducing degenerative changes in cartilage structure or composition.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- CDC. Osteoarthritis. 2011 Available from: http://www.cdc.gov/arthritis/basics/osteoarthritis.htm.
-
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. - PubMed
-
- Peyron JG. Epidemiologic and etiologic approach of osteoarthritis. Semin Arthritis Rheum. 1979;8:288–306. - PubMed
-
- Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–401. - PubMed
-
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

