Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB
- PMID: 22819546
- PMCID: PMC3654796
- DOI: 10.1016/j.jnutbio.2012.03.008
Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB
Abstract
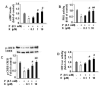
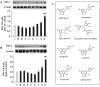
Chronic hyperlipidemia causes β-cell apoptosis and dysfunction, thereby contributing to the pathogenesis of type 2 diabetes (T2D). Thus, searching for agents to promote pancreatic β-cell survival and improve its function could be a promising strategy to prevent and treat T2D. We investigated the effects of kaempferol, a small molecule isolated from ginkgo biloba, on apoptosis and function of β-cells and further determined the mechanism underlying its actions. Kaempferol treatment promoted viability, inhibited apoptosis and reduced caspase-3 activity in INS-1E cells and human islets chronically exposed to palmitate. In addition, kaempferol prevented the lipotoxicity-induced down-regulation of antiapoptotic proteins Akt and Bcl-2. The cytoprotective effects of kaempferol were associated with improved insulin secretion, synthesis, and pancreatic and duodenal homeobox-1 (PDX-1) expression. Chronic hyperlipidemia significantly diminished cyclic adenosine monophosphate (cAMP) production, protein kinase A (PKA) activation, cAMP-responsive element binding protein (CREB) phosphorylation and its regulated transcriptional activity in β-cells, all of which were restored by kaempferol treatment. Disruption of CREB expression by transfection of CREB siRNA in INS-1E cells or adenoviral transfer of dominant-negative forms of CREB in human islets ablated kaempferol protection of β-cell apoptosis and dysfunction caused by palmitate. Incubation of INS-1E cells or human islets with kaempferol for 48h induced PDX-1 expression. This effect of kaempferol on PDX-1 expression was not shared by a host of structurally related flavonoid compounds. PDX-1 gene knockdown reduced kaempferol-stimulated cAMP generation and CREB activation in INS-1E cells. These findings demonstrate that kaempferol is a novel survivor factor for pancreatic β-cells via up-regulating the PDX-1/cAMP/PKA/CREB signaling cascade.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures







References
-
- ADA. Total Prevalence of Diabetes & Pre-diabetes. 2008 http://wwwdiabetesorg/diabetes-statistics/prevalencejsp.
-
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. - PubMed
-
- Stoffers DA. The development of beta-cell mass: recent progress and potential role of GLP-1. Horm Metab Res. 2004;36:811–21. - PubMed
-
- Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–9. - PubMed
-
- Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

