Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion
- PMID: 22819717
- PMCID: PMC5528389
- DOI: 10.1016/j.molonc.2012.06.005
Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion
Abstract
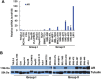
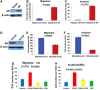
Androgen receptor (AR) activity is associated with cancer development and progression. In hepatocellular carcinoma (HCC), AR contributes to HCC incidence, but the role of AR in HCC cell migration and invasion remains largely unknown. In this study, we found that AR was expressed at high levels in a subgroup of HCC cell lines with high metastatic potential. Experiments using lentiviral overexpression or small hairpin RNA knockdown of AR as well as activation of AR by its ligand indicated that AR activation promoted HCC cell migration and invasion. We also found that AR activation enhanced the expression of a metastasis-promoting gene, ID1, which led to increased HCC cell migration and invasion. An AR antagonist was able to block this process, suggesting that AR activation in AR-positive HCC may be therapeutically inhibited as a potential intervention strategy.
Copyright © 2012 Federation of European Biochemical Societies. All rights reserved.
Figures


References
-
- Groupe d'Etude et de Traitement du Carcinome H'epatocellulaire (GRETCH), 2004. Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology. 40, (6) 1361–1369. - PubMed
-
- Abelev, G.I. , Eraiser, T.L. , 1999. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin. Cancer Biol.. 9, (2) 95–107. - PubMed
-
- Ciarrocchi, A. , Piana, S. , 2011. Inhibitor of DNA binding-1 induces mesenchymal features and promotes invasiveness in thyroid tumour cells. Eur. J. Cancer. 47, (6) 934–945. - PubMed
-
- De Maria, N. , Manno, M. , 2002. Sex hormones and liver cancer. Mol. Cell Endocrinol.. 193, (1–2) 59–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

