HECA-452 is a non-function blocking antibody for isolated sialyl Lewis x adhesion to endothelial expressed E-selectin under flow conditions
- PMID: 22820001
- PMCID: PMC3432728
- DOI: 10.1016/j.jim.2012.07.003
HECA-452 is a non-function blocking antibody for isolated sialyl Lewis x adhesion to endothelial expressed E-selectin under flow conditions
Abstract
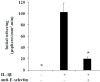
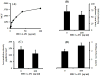
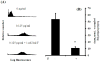
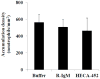
E-selectin, expressed on inflamed endothelium, and sialyl Lewis x (sLe(x)), present on the surface of leukocytes, play a key role in leukocyte-endothelial interactions during leukocyte recruitment to sites of inflammation. HECA-452 is a monoclonal antibody (mAb) that recognizes sLe(x) and is routinely used by investigators from diverse fields who seek to unravel the mechanisms of leukocyte adhesion. The data regarding the ability of HECA-452 to inhibit carbohydrate-mediated leukocyte adhesion to E-selectin remains conflicted, in part due to the presence of a variety of potential E-selectin reactive moieties on leukocytes. Recognizing this, we utilized a complementary approach to gain insight into HECA-452 adhesion assays. Specifically, we used sLe(x) microspheres to investigate the hypothesis that HECA-452 is a non-function blocking mAb for isolated sLe(x) mediated adhesion to endothelial expressed E-selectin. Flow cytometric analysis revealed that HECA-452 recognizes and binds to the sLe(x) microspheres. Perfusion of the sLe(x) microspheres over human umbilical vein endothelial cells (HUVEC) at 1.5 dyn/cm² revealed that the microspheres attach to 4h interleukin (IL)-1β activated HUVEC specifically via E-selectin. Pretreatment of the sLe(x) microspheres with HECA-452 did not influence sLe(x) microsphere initial tethering and accumulation on IL-1β activated HUVEC. Neuraminidase and fucosidase treatments of sLe(x) microspheres revealed that sialic acid and fucose are required for E-selectin binding, whereas HECA-452 recognition of sLe(x) does not depend on the fucose moiety to the extent required for E-selectin recognition. This latter finding suggests there are potential subtle differences between the sLe(x) antigens for E-selectin and HECA-452. Combined, the data indicate that HECA-452 is a non-inhibitor of sLe(x)-mediated adhesion to endothelial expressed E-selectin.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow.Am J Physiol Cell Physiol. 2005 Aug;289(2):C415-24. doi: 10.1152/ajpcell.00289.2004. Epub 2005 Apr 6. Am J Physiol Cell Physiol. 2005. PMID: 15814589
-
Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells.Blood. 2002 Dec 15;100(13):4485-94. doi: 10.1182/blood-2002-06-1799. Epub 2002 Aug 29. Blood. 2002. PMID: 12393554
-
L-selectin ligands expressed by human leukocytes are HECA-452 antibody-defined carbohydrate epitopes preferentially displayed by P-selectin glycoprotein ligand-1.J Immunol. 1999 Nov 1;163(9):5070-8. J Immunol. 1999. PMID: 10528213
-
Design and synthesis of sialyl Lewis(x) mimics as E- and P-selectin inhibitors.Med Res Rev. 2002 Nov;22(6):566-601. doi: 10.1002/med.10018. Med Res Rev. 2002. PMID: 12369089 Review.
-
Interactions between endothelial selectins and cancer cells regulate metastasis.Front Biosci (Landmark Ed). 2011 Jun 1;16(9):3233-51. doi: 10.2741/3909. Front Biosci (Landmark Ed). 2011. PMID: 21622232 Review.
Cited by
-
Staining of E-selectin ligands on paraffin-embedded sections of tumor tissue.BMC Cancer. 2018 May 2;18(1):495. doi: 10.1186/s12885-018-4410-x. BMC Cancer. 2018. PMID: 29716546 Free PMC article.
-
CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions.Am J Physiol Cell Physiol. 2015 Jan 1;308(1):C68-78. doi: 10.1152/ajpcell.00094.2014. Epub 2014 Oct 22. Am J Physiol Cell Physiol. 2015. PMID: 25339657 Free PMC article.
-
An adhesion based approach for the detection of esophageal cancer.Integr Biol (Camb). 2018 Dec 19;10(12):747-757. doi: 10.1039/c8ib00132d. Integr Biol (Camb). 2018. PMID: 30398503 Free PMC article.
-
Simultaneously capturing real-time images in two emission channels using a dual camera emission splitting system: applications to cell adhesion.J Vis Exp. 2013 Sep 4;(79):50604. doi: 10.3791/50604. J Vis Exp. 2013. PMID: 24056855 Free PMC article.
-
PSGL-1 decorated with sialyl Lewisa/x promotes high affinity binding of myeloma cells to P-selectin but is dispensable for E-selectin engagement.Sci Rep. 2024 Jan 19;14(1):1756. doi: 10.1038/s41598-024-52212-2. Sci Rep. 2024. PMID: 38243063 Free PMC article.
References
-
- Alon R, Feizi T, Yuen CT, Fuhlbrigge RC, Springer TA. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J Immunol. 1995;154:5356–66. - PubMed
-
- Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991a;266:14869–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

