A biophysical signature of network affiliation and sensory processing in mitral cells
- PMID: 22820253
- PMCID: PMC3442227
- DOI: 10.1038/nature11291
A biophysical signature of network affiliation and sensory processing in mitral cells
Abstract
One defining characteristic of the mammalian brain is its neuronal diversity. For a given region, substructure, layer or even cell type, variability in neuronal morphology and connectivity persists. Although it is well known that such cellular properties vary considerably according to neuronal type, the substantial biophysical diversity of neurons of the same morphological class is typically averaged out and ignored. Here we show that the amplitude of hyperpolarization-evoked sag of membrane potential recorded in olfactory bulb mitral cells is an emergent, homotypic property of local networks and sensory information processing. Simultaneous whole-cell recordings from pairs of cells show that the amount of hyperpolarization-evoked sag potential and current (Ih) is stereotypic for mitral cells belonging to the same glomerular circuit. This is corroborated by a mosaic, glomerulus-based pattern of expression of the HCN2 (hyperpolarization-activated cyclic nucleotide-gated channel 2) subunit of the Ih channel. Furthermore, inter-glomerular differences in both membrane potential sag and HCN2 protein are diminished when sensory input to glomeruli is genetically and globally altered so that only one type of odorant receptor is universally expressed. Population diversity in this intrinsic property therefore reflects differential expression between local mitral cell networks processing distinct odour-related information.
Figures
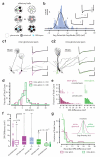


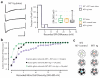
Comment in
-
Neuroscience: Circuits drive cell diversity.Nature. 2012 Aug 16;488(7411):289-90. doi: 10.1038/488289a. Nature. 2012. PMID: 22895331 No abstract available.
References
-
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. - PubMed
-
- Brochtrup A, Hummel T. Olfactory map formation in the Drosophila brain: genetic specificity and neuronal variability. Curr Opin Neurobiol. 2011;21:85–92. - PubMed
-
- Reyes A, et al. Target-cell specific facilitation and depression in neocortical networks. Nature Neuroscience. 1998;1:279–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

