Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain
- PMID: 22820296
- PMCID: PMC3454487
- DOI: 10.1016/j.brainres.2012.06.055
Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain
Abstract
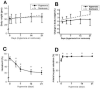
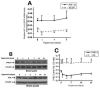
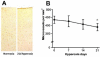
Normal brain function is dependent on continuous and controlled oxygen delivery. Chronic moderate hypoxia leads to angiogenesis, suggesting a modulatory role for oxygen in determining capillary density. The objective of this study was to determine physiologic and brain angiogenic adaptational changes during chronic moderate normobaric hyperoxia in mice. Four-month old C56BL/6J mice were kept in a normobaric chamber at 50% O(2) for up to 3 weeks. Normoxic littermates were kept in the same room outside the chamber. Freshly collected or fixed brain specimens were analyzed by RT-PCR, Western blot analysis and immunohistochemistry. Results show accumulation of hypoxia inducible factors 1 and 2α (HIF-1 and 2α), and increased expression of erythropoietin (EPO), cyclooxygenase-2 (COX-2) and angiopoietin-2 (Ang-2). Conversely, vascular endothelial growth factor (VEGF), and VEGF receptor-2 (KDR/Flk-1), Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) and prolylhydroxylase-2 (PHD-2) expressions were decreased. VEGF mRNA level was diminished but there was no change in HIF-1α mRNA and von Hippel Lindau E3 ubiquitin ligase (VHL) protein expression. Microvascular density was significantly diminished by the end of the 3rd week of hyperoxia. Overall, our results are: (1) increased expression of the potent neuroprotective molecule, EPO; (2) diminished expression of the potent angiogenic factor, VEGF; and (3) decreased microvascular density. We can, therefore, conclude that brain microvascular density can be controlled by HIF-independent mechanisms, and that brain capillary density is a continuously adjusted variable with tissue oxygen availability as one of the controlling modulators.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
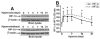
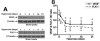
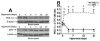
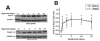
References
-
- Ahmad A, Ahmad S, Chang LY, Schaack J, White CW. Endothelial Akt activation by hyperoxia: role in cell survival. Free Radic. Biol. Med. 2006;40:1108–1118. - PubMed
-
- Allen BW, Demchenko IT, Piantadosci CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J. Appl. Physiol. 2009;106:662–667. - PubMed
-
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spieglman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. - PubMed
-
- Bartesaghi S, Marinovich M, Corsini E, Galli CL, Viviani B. Erythropoietin: a novel neuroprotective cytokine. Neurotoxicology. 2005;26:923–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

