Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis
- PMID: 22820377
- PMCID: PMC3606816
- DOI: 10.1038/ncb2546
Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis
Abstract
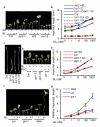
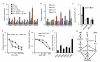
Brassinosteroid and gibberellin promote many similar developmental responses in plants; however, their relationship remains unclear. Here we show that BR and GA act interdependently through a direct interaction between the BR-activated BZR1 and GA-inactivated DELLA transcription regulators. GA promotion of cell elongation required BR signalling, whereas BR or active BZR1 suppressed the GA-deficient dwarf phenotype. DELLAs directly interacted with BZR1 and inhibited BZR1-DNA binding both in vitro and in vivo. Genome-wide analysis defined a BZR1-dependent GA-regulated transcriptome, which is enriched with light-regulated genes and genes involved in cell wall synthesis and photosynthesis/chloroplast function. GA promotion of hypocotyl elongation requires both BZR1 and the phytochrome-interacting factors (PIFs), as well as their common downstream targets encoding the PRE-family helix-loop-helix factors. The results demonstrate that GA releases DELLA-mediated inhibition of BZR1, and that the DELLA-BZR1-PIF4 interaction defines a core transcription module that mediates coordinated growth regulation by GA, BR and light signals.
Figures



Comment in
-
Brassinosteroids, gibberellins and light-mediated signalling are the three-way controls of plant sprouting.Nat Cell Biol. 2012 Aug;14(8):788-90. doi: 10.1038/ncb2551. Nat Cell Biol. 2012. PMID: 22854813
References
-
- Depuydt S, Hardtke CS. Hormone signalling crosstalk in plant growth regulation. Curr Biol. 2011;21:R365–373. - PubMed
-
- Szekeres M, et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. - PubMed
-
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

