The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters: Case studies
- PMID: 22820463
- PMCID: PMC3499303
- DOI: 10.4161/mabs.20814
The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters: Case studies
Abstract
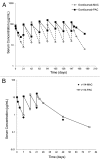
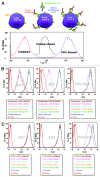
To interpret pharmacokinetic (PK) data of biotherapeutics, it is critical to understand which drug species is being measured by the PK assay. For therapeutic antibodies, it is generally accepted that "free" circulating antibodies are the pharmacologically active form needed to determine the PK/pharmacodynamic (PD) relationship, safety margin calculations, and dose projections from animals to humans and the eventual characterization of the exposure in the clinic. However, "total" drug may be important in evaluating the dynamic interaction between the drug and the target, as well as the total drug exposure. In the absence of or with low amounts of soluble ligand/shed receptor, total and free drug species are often equivalent and their detection is less sensitive to assay formats or reagent choices. In contrast, in the presence of a significant amount of ligand, assay design and characterization of assay reagents are critical to understanding the PK profiles. Here, we present case studies where different assay formats affected measured PK profiles and data interpretation. The results from reagent characterizations provide a potential explanation for the observed discrepancies and highlight the importance of reagent characterization in understanding which drug species are being measured to accurately interpret PK parameters.
Figures



References
-
- Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;13:99–110. doi: 10.1208/s12248-011-9251-3. - DOI - PMC - PubMed
-
- Friedberg JW, Vose JM, Kahl BS, Brunvand MW, Goy A, Kasamon YL, et al. A phase I study of PRO131921, a novel anti-CD20 monoclonal antibody in patients with relapsed/refractory CD20_ indolent NHL: Correlation between clinical responses and AUC pharmacokinetics. ASH Annual Meeting. New Orleans, LA: Blood, 2009:3742. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
