Na+ and K+ ion imbalances in Alzheimer's disease
- PMID: 22820549
- PMCID: PMC3444663
- DOI: 10.1016/j.bbadis.2012.07.004
Na+ and K+ ion imbalances in Alzheimer's disease
Abstract
Alzheimer's disease (AD) is associated with impaired glutamate clearance and depressed Na(+)/K(+) ATPase levels in AD brain that might lead to a cellular ion imbalance. To test this hypothesis, [Na(+)] and [K(+)] were analyzed in postmortem brain samples of 12 normal and 16 AD individuals, and in cerebrospinal fluid (CSF) from AD patients and matched controls. Statistically significant increases in [Na(+)] in frontal (25%) and parietal cortex (20%) and in cerebellar [K(+)] (15%) were observed in AD samples compared to controls. CSF from AD patients and matched controls exhibited no differences, suggesting that tissue ion imbalances reflected changes in the intracellular compartment. Differences in cation concentrations between normal and AD brain samples were modeled by a 2-fold increase in intracellular [Na(+)] and an 8-15% increase in intracellular [K(+)]. Since amyloid beta peptide (Aβ) is an important contributor to AD brain pathology, we assessed how Aβ affects ion homeostasis in primary murine astrocytes, the most abundant cells in brain tissue. We demonstrate that treatment of astrocytes with the Aβ 25-35 peptide increases intracellular levels of Na(+) (~2-3-fold) and K(+) (~1.5-fold), which were associated with reduced levels of Na(+)/K(+) ATPase and the Na(+)-dependent glutamate transporters, GLAST and GLT-1. Similar increases in astrocytic Na(+) and K(+) levels were also caused by Aβ 1-40, but not by Aβ 1-42 treatment. Our study suggests a previously unrecognized impairment in AD brain cell ion homeostasis that might be triggered by Aβ and could significantly affect electrophysiological activity of brain cells, contributing to the pathophysiology of AD.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

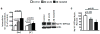


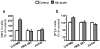
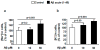
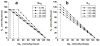

References
-
- Pike CJ, Cummings BJ, Monzavi R, Cotman CW. Beta-amyloid-induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer’s disease. Neuroscience. 1994;63:517–531. - PubMed
-
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. - PubMed
-
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–416. - PubMed
-
- Hattori N, Kitagawa K, Higashida T, Yagyu K, Shimohama S, Wataya T, Perry G, Smith MA, Inagaki C. CI-ATPase and Na+/K(+)-ATPase activities in Alzheimer’s disease brains. Neurosci Lett. 1998;254:141–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AG026276/AG/NIA NIH HHS/United States
- NS34709/NS/NINDS NIH HHS/United States
- P50 AG008671/AG/NIA NIH HHS/United States
- 5P50 AG008671/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- P01AG03991/AG/NIA NIH HHS/United States
- DK64959/DK/NIDDK NIH HHS/United States
- R01 NS034709/NS/NINDS NIH HHS/United States
- P50AG05681/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- R01 DK064959/DK/NIDDK NIH HHS/United States
- R01 HL058984/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

