Characterization of peripheral and mucosal immune responses in rhesus macaques on long-term tenofovir and emtricitabine combination antiretroviral therapy
- PMID: 22820802
- PMCID: PMC3494791
- DOI: 10.1097/QAI.0b013e318266be53
Characterization of peripheral and mucosal immune responses in rhesus macaques on long-term tenofovir and emtricitabine combination antiretroviral therapy
Abstract
Background: The goal of antiretroviral therapy (ART) is to suppress virus replication to limit immune system damage. Some have proposed combining ART with immune therapies to boost antiviral immunity. For this to be successful, ART must not impair physiological immune function.
Methods: We studied the impact of ART (tenofovir and emtricitabine) on systemic and mucosal immunity in uninfected and simian immunodeficiency (SIV)-infected Chinese rhesus macaques. Subcutaneous ART was initiated 2 weeks after tonsillar inoculation with SIVmac239.
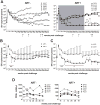
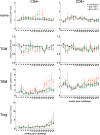
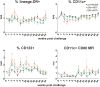
Results: There was no evidence of immune dysregulation as a result of ART in either infected or uninfected animals. Early virus-induced alterations in circulating immune cell populations (decreased central memory T cells and myeloid dendritic cells) were detected, but normalized shortly after ART initiation. ART-treated animals showed marginal SIV-specific T-cell responses during treatment, which increased after ART discontinuation. Elevated expression of CXCL10 in oral, rectal, and blood samples and APOBEC3G mRNA in oral and rectal tissues was observed during acute infection and was down regulated after starting ART. ART did not impact the ability of the animals to respond to tonsillar application of polyICLC with increased CXCL10 expression in oral fluids and CD80 expression on blood myeloid dendritic cells.
Conclusion: Early initiation of ART prevented virus-induced damage and did not impede mucosal or systemic immune functions.
Conflict of interest statement
None of the authors has a conflict of interest with this research. None of the material in this manuscript has been published or is under consideration elsewhere.
Figures


Similar articles
-
IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques.Blood. 2011 Sep 1;118(9):2520-9. doi: 10.1182/blood-2011-05-351155. Epub 2011 Jul 14. Blood. 2011. PMID: 21757617 Free PMC article.
-
Increased CD4+ T cell levels during IL-7 administration of antiretroviral therapy-treated simian immunodeficiency virus-positive macaques are not dependent on strong proliferative responses.J Immunol. 2010 Aug 1;185(3):1650-9. doi: 10.4049/jimmunol.0902626. Epub 2010 Jul 9. J Immunol. 2010. PMID: 20622118 Free PMC article.
-
A highly intensified ART regimen induces long-term viral suppression and restriction of the viral reservoir in a simian AIDS model.PLoS Pathog. 2012;8(6):e1002774. doi: 10.1371/journal.ppat.1002774. Epub 2012 Jun 21. PLoS Pathog. 2012. PMID: 22737073 Free PMC article.
-
Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques.Retrovirology. 2006 Dec 21;3:97. doi: 10.1186/1742-4690-3-97. Retrovirology. 2006. PMID: 17184540 Free PMC article.
-
Emtricitabine/tenofovir disoproxil fumarate.Drugs R D. 2004;5(3):160-1. doi: 10.2165/00126839-200405030-00004. Drugs R D. 2004. PMID: 15139777 Review.
Cited by
-
Robust suppression of env-SHIV viremia in Macaca nemestrina by 3-drug ART is independent of timing of initiation during chronic infection.J Med Primatol. 2013 Oct;42(5):237-46. doi: 10.1111/jmp.12060. J Med Primatol. 2013. PMID: 24025078 Free PMC article.
-
Considerations in the development of nonhuman primate models of combination antiretroviral therapy for studies of AIDS virus suppression, residual virus, and curative strategies.Curr Opin HIV AIDS. 2013 Jul;8(4):262-72. doi: 10.1097/COH.0b013e328361cf40. Curr Opin HIV AIDS. 2013. PMID: 23698559 Free PMC article. Review.
-
Persistent Viral Reservoirs in Lymphoid Tissues in SIV-Infected Rhesus Macaques of Chinese-Origin on Suppressive Antiretroviral Therapy.Viruses. 2019 Jan 27;11(2):105. doi: 10.3390/v11020105. Viruses. 2019. PMID: 30691203 Free PMC article.
-
Residual immune dysregulation syndrome in treated HIV infection.Adv Immunol. 2013;119:51-83. doi: 10.1016/B978-0-12-407707-2.00002-3. Adv Immunol. 2013. PMID: 23886064 Free PMC article. Review.
-
PolyICLC Exerts Pro- and Anti-HIV Effects on the DC-T Cell Milieu In Vitro and In Vivo.PLoS One. 2016 Sep 7;11(9):e0161730. doi: 10.1371/journal.pone.0161730. eCollection 2016. PLoS One. 2016. PMID: 27603520 Free PMC article.
References
-
- Desrosiers RC. The simian immunodeficiency viruses. Ann Rev Immunol. 1990;8:557–578. - PubMed
-
- Desrosiers RC. Non-human primate models for AIDS vaccines. Aids. 1995;9(Suppl A):S137–141. - PubMed
-
- Van Rompay KK. Evaluation of antiretrovirals in animal models of HIV infection. Antiviral Res. 2010 Jan;85(1):159–175. - PubMed
-
- Van Rompay KK. Antiretroviral drug studies in nonhuman primates: a valid animal model for innovative drug efficacy and pathogenesis experiments. AIDS Rev. 2005 Apr-Jun;7(2):67–83. - PubMed
-
- Franchini G, Nacsa J, Hel Z, Tryniszewska E. Immune intervention strategies for HIV-1 infection of humans in the SIV macaque model. Vaccine. 2002 Dec 19;20(Suppl 4):A52–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G20 RR019607/RR/NCRR NIH HHS/United States
- G20 RR018397/RR/NCRR NIH HHS/United States
- G20 RR021381/RR/NCRR NIH HHS/United States
- 1G20RR21381/RR/NCRR NIH HHS/United States
- DE018293/DE/NIDCR NIH HHS/United States
- 1CO6RR012112-01/CO/NCI NIH HHS/United States
- R01 DE018293/DE/NIDCR NIH HHS/United States
- RR000164/RR/NCRR NIH HHS/United States
- 1G20RR013466-01/RR/NCRR NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- G20 RR022760/RR/NCRR NIH HHS/United States
- G20 RR013466/RR/NCRR NIH HHS/United States
- 1G20RR016930-01/RR/NCRR NIH HHS/United States
- G20 RR019628/RR/NCRR NIH HHS/United States
- 1G20RR22760/RR/NCRR NIH HHS/United States
- G20 RR016930/RR/NCRR NIH HHS/United States
- 1G20RR018397-01/RR/NCRR NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- 1G20RR019607-01/RR/NCRR NIH HHS/United States
- 1G20RR019628-01/RR/NCRR NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

