Formation and migration of neural crest cells in the vertebrate embryo
- PMID: 22820859
- PMCID: PMC3425661
- DOI: 10.1007/s00418-012-0999-z
Formation and migration of neural crest cells in the vertebrate embryo
Abstract
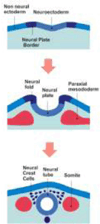
The neural crest is a stem cell population, unique to vertebrates, that gives rise to a vast array of derivatives, ranging from peripheral ganglia to the facial skeleton. This population is induced in the early embryo at the border of the neural plate, which will form the central nervous system (CNS). After neural tube closure, neural crest cells depart from the dorsal CNS via an epithelial to mesenchymal transition (EMT), forming a migratory mesenchymal cell type that migrates extensive to diverse locations in the embryo. Using in vivo loss-of-function approaches and cis-regulatory analysis coupled with live imaging, we have investigated the gene regulatory network that mediates formation of this fascinating cell type. The results show that a combination of transcriptional inputs and epigenetic modifiers control the timing of onset of neural crest gene expression. This in turn leads to the EMT process that produces this migratory cell population.
Figures




References
-
- Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

