Plant hormonal regulation of nitrogen-fixing nodule organogenesis
- PMID: 22820920
- PMCID: PMC3887813
- DOI: 10.1007/s10059-012-0131-1
Plant hormonal regulation of nitrogen-fixing nodule organogenesis
Abstract
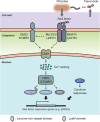
Legumes have evolved symbiotic interactions with rhizobial bacteria to efficiently utilize nitrogen. Recent progress in symbiosis has revealed several key components of host plants required for nitrogen-fixing nodule organogenesis, in which complicated metabolic and signaling pathways in the host plant are reprogrammed to generate nodules in the cortex upon perception of the rhizobial Nod factor. Following the recognition of Nod factors, plant hormones are likely to be essential throughout nodule organogenesis for integration of developmental and environmental signaling cues into nodule development. Here, we review the molecular events involved in plant hormonal regulation and signaling cross-talk for nitrogen-fixing nodule development, and discuss how these signaling networks are integrated into Nod factor-mediated signaling during plant-microbe interactions.
Figures


References
-
- Ane J.M., Kiss G.B., Riely B.K., Penmetsa R.V., Oldroyd G.E., Ayax C., Levy J., Debelle F., Baek J.M., Kalo P., et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–1367. - PubMed
-
- Arrighi J.F., Barre A., Ben Amor B., Bersoult A., Soriano L.C., Mirabella R., de Carvalho-Niebel F., Journet E.P., Gherardi M., Huguet T., et al. The Medicago truncatula lysin [corrected] motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006;142:265–279. - PMC - PubMed
-
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

