Lucinactant attenuates pulmonary inflammatory response, preserves lung structure, and improves physiologic outcomes in a preterm lamb model of RDS
- PMID: 22821059
- PMCID: PMC3888789
- DOI: 10.1038/pr.2012.96
Lucinactant attenuates pulmonary inflammatory response, preserves lung structure, and improves physiologic outcomes in a preterm lamb model of RDS
Abstract
Background: Acute inflammatory responses to supplemental oxygen and mechanical ventilation have been implicated in the pathophysiological sequelae of respiratory distress syndrome (RDS). Although surfactant replacement therapy (SRT) has contributed to lung stability, the effect on lung inflammation is inconclusive. Lucinactant contains sinapultide (KL4), a novel synthetic peptide that functionally mimics surfactant protein B, a protein with anti-inflammatory properties. We tested the hypothesis that lucinactant may modulate lung inflammatory response to mechanical ventilation in the management of RDS and may confer greater protection than animal-derived surfactants.
Methods: Preterm lambs (126.8 ± 0.2 SD d gestation) were randomized to receive lucinactant, poractant alfa, beractant, or no surfactant and studied for 4 h. Gas exchange and pulmonary function were assessed serially. Lung inflammation biomarkers and lung histology were assessed at termination.
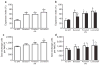
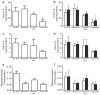
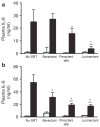
Results: SRT improved lung compliance relative to no SRT without significant difference between SRT groups. Lucinactant attenuated lung and systemic inflammatory response, supported oxygenation at lower ventilatory requirements, and preserved lung structural integrity to a greater degree than either no SRT or SRT with poractant alfa or beractant.
Conclusion: These data suggest that early intervention with lucinactant may more effectively mitigate pulmonary pathophysiological sequelae of RDS than the animal-derived surfactants poractant alfa or beractant.
Figures
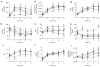

Similar articles
-
Comparison of poractant alfa and lyophilized lucinactant in a preterm lamb model of acute respiratory distress.Pediatr Res. 2012 Jul;72(1):32-7. doi: 10.1038/pr.2012.46. Pediatr Res. 2012. PMID: 22465908
-
A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome.Pediatrics. 2005 Apr;115(4):1030-8. doi: 10.1542/peds.2004-2231. Pediatrics. 2005. PMID: 15805381 Clinical Trial.
-
Pulmonary distribution of lucinactant and poractant alfa and their peridosing hemodynamic effects in a preterm lamb model of respiratory distress syndrome.Pediatr Res. 2010 Sep;68(3):193-8. doi: 10.1203/PDR.0b013e3181eaff66. Pediatr Res. 2010. PMID: 20531255
-
Lucinactant: in neonatal respiratory distress syndrome.Treat Respir Med. 2005;4(2):139-45; discussion 146-7. doi: 10.2165/00151829-200504020-00008. Treat Respir Med. 2005. PMID: 15813666 Review.
-
Lucinactant for the prevention of respiratory distress syndrome in premature infants.Expert Rev Clin Pharmacol. 2013 Mar;6(2):115-21. doi: 10.1586/ecp.12.80. Expert Rev Clin Pharmacol. 2013. PMID: 23473590 Review.
Cited by
-
Persistent pulmonary hypertension of the newborn.Matern Health Neonatol Perinatol. 2015 Jun 3;1:14. doi: 10.1186/s40748-015-0015-4. eCollection 2015. Matern Health Neonatol Perinatol. 2015. PMID: 27057331 Free PMC article.
-
Early Immunomodulatory Effects of Different Natural Surfactant Preparations in Preterms With Respiratory Distress.Front Pediatr. 2022 Mar 18;10:845780. doi: 10.3389/fped.2022.845780. eCollection 2022. Front Pediatr. 2022. PMID: 35372166 Free PMC article.
-
Surfactant treatment before first breath for respiratory distress syndrome in preterm lambs: comparison of a peptide-containing synthetic lung surfactant with porcine-derived surfactant.Drug Des Devel Ther. 2013 Aug 29;7:905-16. doi: 10.2147/DDDT.S47270. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24039400 Free PMC article.
-
Perfluorochemical-facilitated plasminogen activator delivery to the airways: A novel treatment for inhalational smoke-induced acute lung injury.Clin Transl Med. 2020 Jan;10(1):258-274. doi: 10.1002/ctm2.26. Clin Transl Med. 2020. PMID: 32508014 Free PMC article.
-
Maintenance of human amnion epithelial cell phenotype in pulmonary surfactant.Stem Cell Res Ther. 2014 Sep 4;5(5):107. doi: 10.1186/scrt495. Stem Cell Res Ther. 2014. PMID: 25189170 Free PMC article.
References
-
- Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001;13:124–9. - PubMed
-
- Lodha A, Asztalos E, Moore AM. Cytokine levels in neonatal necrotizing enterocolitis and long-term growth and neurodevelopment. Acta Paediatr. 2010;99:338–43. - PubMed
-
- American Academy of Pediatrics – Committee on fetus and newborn and Canadian Paediatric Society – Fetus and newborn committee. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

