Role of EphA/ephrin--a signaling in the development of topographic maps in mouse corticothalamic projections
- PMID: 22821544
- PMCID: PMC3511615
- DOI: 10.1002/cne.23195
Role of EphA/ephrin--a signaling in the development of topographic maps in mouse corticothalamic projections
Abstract
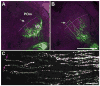
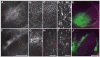
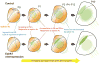
Corticothalamic (CT) feedback outnumbers thalamocortical projections and regulates sensory information processing at the level of the thalamus. It is well established that EphA7, a member of EphA receptor family, is involved in the topographic mapping of CT projections. The aim of the present study was to dissect the precise impact of EphA7 on each step of CT growth. We used in utero electroporation-mediated EphA7 overexpression in developing somatosensory CT axons to dissect EphA7/ephrin-A-dependent mechanisms involved in regulating both initial targeting and postnatal growth of the CT projections. Our data revealed that topographic maps of cortical afferents in the ventrobasal complex and medial part of the posterior complex in the thalamus become discernible shortly after birth and are fully established by the second postnatal week. This process starts with the direct ingrowth of the CT axons to the designated areas within target thalamic nuclei and by progressive increase of axonal processes in the terminal zones. Large-scale overproduction and elimination of exuberant widespread axonal branches outside the target zone was not observed. Each developmental event was coordinated by spatially and temporally different responsiveness of CT axons to the ephrin-A gradient in thalamic nuclei, as well as by the matching levels of EphA7 in CT axons and ephrin-As in thalamic nuclei. These results support the concept that the topographic connections between the maps in the cerebral cortex and corresponding thalamic nuclei are genetically prespecified to a large extent, and established by precise spatiotemporal molecular mechanisms that involve the Eph family of genes.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
Figures



References
-
- Alloway KD, Hoffer ZS, Hoover JE. Quantitative comparisons of corticothalamic topography within the ventrobasal complex and the posterior nucleus of the rodent thalamus. Brain Res. 2003;968(1):54–68. - PubMed
-
- Bielle F, Marcos-Mondejar P, Leyva-Diaz E, Lokmane L, Mire E, Mailhes C, Keita M, Garcia N, Tessier-Lavigne M, Garel S, Lopez-Bendito G. Emergent growth cone responses to combinations of Slit1 and Netrin 1 in thalamocortical axon topography. Curr Biol. 2011;21(20):1748–1755. - PubMed
-
- Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59(1):82–94. - PubMed
-
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10(5):588–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

