Biglycan: a multivalent proteoglycan providing structure and signals
- PMID: 22821552
- PMCID: PMC3527886
- DOI: 10.1369/0022155412456380
Biglycan: a multivalent proteoglycan providing structure and signals
Abstract
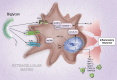
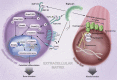
Research over the past few years has provided fascinating results indicating that biglycan, besides being a ubiquitous structural component of the extracellular matrix (ECM), may act as a signaling molecule. Proteolytically released from the ECM, biglycan acts as a danger signal signifying tissue stress or injury. As a ligand of innate immunity receptors and activator of the inflammasome, biglycan stimulates multifunctional proinflammatory signaling linking the innate to the adaptive immune response. By clustering several types of receptors on the cell surface and orchestrating their downstream signaling events, biglycan is capable to autonomously trigger sterile inflammation and to potentiate the inflammatory response to microbial invasion. Besides operating in a broad biological context, biglycan also displays tissue-specific affinities to certain receptors and structural components, thereby playing a crucial role in bone formation, muscle integrity, and synapse stability at the neuromuscular junction. This review attempts to provide a concise summary of recent data regarding the involvement of biglycan in the regulation of inflammation and the musculoskeletal system, pointing out both a signaling and a structural role for this proteoglycan. The potential of biglycan as a novel therapeutic target or agent for the treatment of inflammatory diseases and skeletal muscular dystrophies is also addressed.
Conflict of interest statement
Figures
Similar articles
-
Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors.J Biol Chem. 2009 Sep 4;284(36):24035-48. doi: 10.1074/jbc.M109.014266. Epub 2009 Jul 15. J Biol Chem. 2009. PMID: 19605353 Free PMC article.
-
Bimodal role of NADPH oxidases in the regulation of biglycan-triggered IL-1β synthesis.Matrix Biol. 2016 Jan;49:61-81. doi: 10.1016/j.matbio.2015.12.005. Epub 2015 Dec 12. Matrix Biol. 2016. PMID: 26689330 Free PMC article.
-
The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages.J Clin Invest. 2005 Aug;115(8):2223-33. doi: 10.1172/JCI23755. Epub 2005 Jul 14. J Clin Invest. 2005. PMID: 16025156 Free PMC article.
-
Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development.Semin Cancer Biol. 2020 May;62:31-47. doi: 10.1016/j.semcancer.2019.07.026. Epub 2019 Aug 11. Semin Cancer Biol. 2020. PMID: 31412297 Review.
-
Breaking down chronic inflammatory diseases: the role of biglycan in promoting a switch between inflammation and autophagy.FEBS J. 2019 Aug;286(15):2965-2979. doi: 10.1111/febs.14791. Epub 2019 Feb 27. FEBS J. 2019. PMID: 30776184 Review.
Cited by
-
Drosophila hemocytes recognize lymph gland tumors of mxc mutants and activate the innate immune pathway in a reactive oxygen species-dependent manner.Biol Open. 2022 Nov 1;11(11):bio059523. doi: 10.1242/bio.059523. Epub 2022 Nov 3. Biol Open. 2022. PMID: 36226812 Free PMC article.
-
Proteoglycans in Articular Cartilage and Their Contribution to Chondral Injury and Repair Mechanisms.Int J Mol Sci. 2023 Jun 28;24(13):10824. doi: 10.3390/ijms241310824. Int J Mol Sci. 2023. PMID: 37446002 Free PMC article. Review.
-
Proteoglycans as potential biomarkers in odontogenic tumors.J Oral Maxillofac Pathol. 2018 Jan-Apr;22(1):98-103. doi: 10.4103/jomfp.JOMFP_151_17. J Oral Maxillofac Pathol. 2018. PMID: 29731564 Free PMC article. Review.
-
Serum Decorin and Biglycan as Potential Biomarkers to Predict PPROM in Early Gestation.Reprod Sci. 2020 Aug;27(8):1620-1626. doi: 10.1007/s43032-020-00192-9. Reprod Sci. 2020. PMID: 32436194 Free PMC article.
-
Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis.J Am Soc Nephrol. 2014 Jul;25(7):1387-400. doi: 10.1681/ASN.2014010117. Epub 2014 Apr 24. J Am Soc Nephrol. 2014. PMID: 24762401 Free PMC article. Review.
References
-
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. 2002. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 16:673–680 - PubMed
-
- Ameye L, Young MF. 2002. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 12:107R–1016R - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

